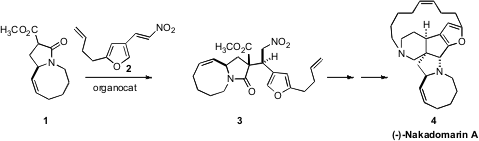
(-)-Nakadomarin A (4), isolated from the sponge Amphimedon sp. off the
coast of Okinama, shows interesting antifungal and antibacterial activity. The
key step in the total synthesis of 4 reported
(J. Am. Chem. PMID:25429455 Soc. 2009, 131, 16632.
DOI: 10.1021/ja908399s)
by Darren J. Dixon of the University of Oxford was the diastereoselective addition
of the enantiomerically-pure ester 1 to the prochiral nitroalkene 2.
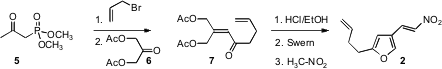
The assembly of 2 began with the linchpin ketophosphonate 5.
Alkylation of the dianion of 5 with allyl bromide followed by direct
condensation of the resulting monoanion with the diacetate 6 gave 7.
On exposure to aqueous acid, 7 cyclized to the
furan. 2-Chloro-3-methoxypyridin-4-amine site Price of (6Z,9Z)-18-Bromooctadeca-6,9-diene Oxidation of the liberated primary alcohol followed by condensation with nitromethane then
completed the preparation of 2.
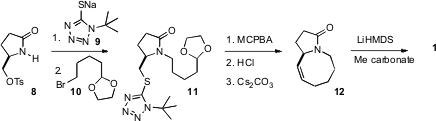
The starting material for the synthesis of 1 was the
enantiomerically-pure pyroglutamate derivative 8. Sulfide displacement
followed by N-alkylation with the bromide 10 delivered 11.
Oxidation followed by deprotection then set the stage for the intramolecular
Julia-Kocienski cyclization, that gave 12 with the expected (eight-membered
ring) high geometric control.
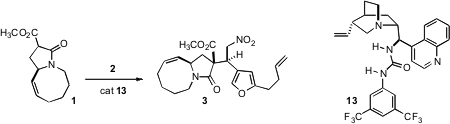
Addition of the ester 1 to Michael acceptors proceeded across the open
face of the lactam, but it was still necessary to control the face of the nitro
alkene 2 to which the lactam anion added. Catalysis of the addition with
the urea 13 delivered 3 with 10:1 diasterocontrol.
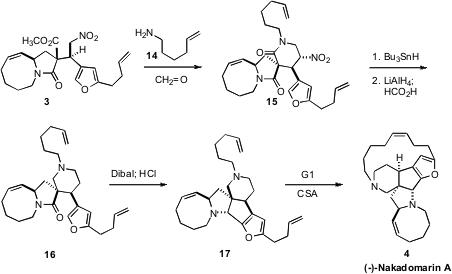
Mannich condensation of the nitroalkane 3 with formaldehyde and the
amine 14 gave the bis-lactam 15, conveniently as a single
diastereomer. After free radical removal of the nitro group, it was necessary to
achieve selective reduction of the
δ-lactam in the presence of the
γ-lactam. Low temperature
LiAlH4 was found to be effective. Direct reduction of the resulting
hemiaminal with formic acid led to the monolactam 16. The hemiaminal from
monoreduction of 16 was found to be unstable and sensitive to over-reduction.
Nevertheless, exposure of 16 to Dibal at low temperature followed by acid-mediated
cyclization delivered the diamine 17.
Cyclization of the free base of 17 with the first generation Grubbs catalyst
gave (-)-Nakadomarin A (4) as the minor component of a 40:60 Z/E mixture. Carrying
out the cyclization on the camphorsulfonate salt improved the ratio to 63:37
Z/E.