There are two criteria for judging any total synthesis: the importance of the molecule that has been prepared, and the creativity evidenced in the synthetic route. 341-58-2 Data Sheet When the natural product has only two rings, as with borrelidin1, the standards are even higher. The enantioselective total synthesis of borrelidin by Jim Morken of the University of North Carolina (J. Am. Chem. Soc. 181434-36-6 Chemical name 2003, 125, 1458.DOI: 10.1021/ja028941u)more than exceeds those standards. PMID:23935843
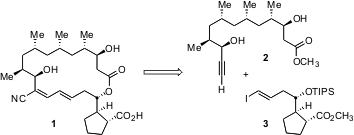
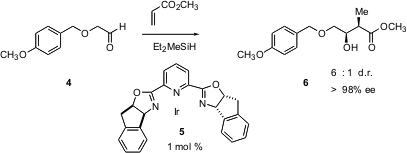
Borrelidin 1 has attracted attention because it inhibits angiogenesis, and so potentially blocks tumor growth, with an IC50 of 0.8 nM. Retrosynthetic analysis of 1 led the investigators to the prospective intermediates 2 and 3. To assemble these two fragments, they interatively deployed the elegant enantio- and diastereoselective intermolecular reductive ester aldol condensation that they had recently developed. This transformation is exemplified by the homologation of 4 to 6 catalyzed by the enantiomerically-pure Ir complex 5.
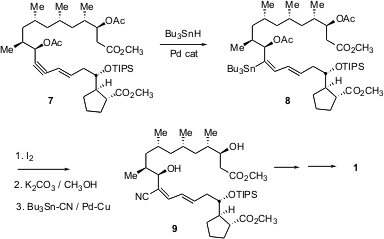
The final stages of the synthesis illustrate both the power and the current limitations of transition-metal mediated C-C bond formation. Coupling of 2 and 3 led to the ene-yne 7. Pd-mediated hydrostannylation of the alkyne proceeded with high geometric control, but tended to give the undesired regioisomer. The authors found that with the acetate, the ratio could be improved to 1 : 1. Iodination followed by Stille coupling then gave the dienyl nitrile9.