The correct assignment of relative configuration for portions of a complex
structure that are remote one from another can present substantial difficulties. PMID:25269910
This was brought home in the course of the synthesis of (+)-Vannusal (3) described
(Angew. Chem. Int. 4,4′-Dibromo-2,2′-bipyridine uses Ed. 2009, 48, 5642,
DOI: 10.1002/anie.200902028;
5648, DOI: 10.1002/anie.200902029)
by K. C. Nicolau of Scripps/La Jolla. Formula of 4-Bromoisoxazol-3-amine In fact, they prepared several alternative
diastereomers, including the originally assigned structure, before finally
coming to 3, the spectra of which matched those of the natural product.
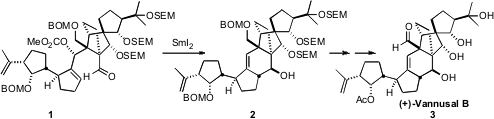
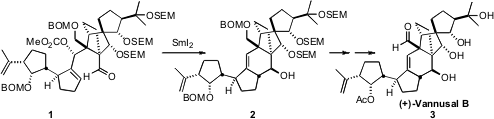
Their synthetic strategy was based on the late-stage convergent coupling of
the aldehyde 13 with the iodide 19, leading to 1. The
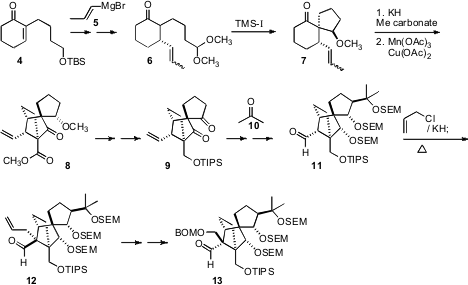
preparation of 13 began with conjugate addition of the 1-propenyl
Grignard reagent 5 to the
cyclohexenone 4. Deprotection, oxidation
and acetal formation led to 6, that cyclized with high diastereocontrol
to 7. Carbomethoxylation of the ketone followed by
Mn(OAc)3
cyclization delivered the highly strained norbornane 8 as a single
diastereomer. Condensation of the derived ketone 9 with acetone (10)
followed by reduction set the three remaining ternary stereogenic centers of
13. O-Alkylation of the aldehyde 11 followed by
Claisen rearrangement
established the alkylated quaternary center. Functional group manipulation then
converted 12 into 13.
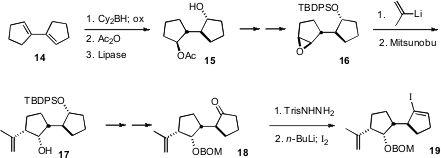
The preparation of the iodide 19 began with the diene 14.
Hydroboration followed by acetylation provided the meso diol. Enzymatic
hydrolysis proceeded with high enantioselectivity, to give 15. Opening of
the epoxide 16 with 2-propenyl lithium gave the trans alcohol, that was
converted to the requisite cis alcohol 17 by
Mitsunobu esterification followed by hydrolysis.
Shapiro iodination of 18 then delivered 19.
The iodide 19 was enantiomerically pure, but the aldehyde 13
was racemic, so coupling of the two led to 1 and its diastereomer. The
cyclization of 1 with
SmI2 proceeded with remarkable
diastereocontrol, to give the desired 2 directly. Deprotection and
oxidation then completed the synthesis of (+)-Vannusal B (3).
It is noteworthy that throughout this synthesis, the radicals
AZADO (20)
and 1-Me-AZADO (21), developed by Professor Iwabuchi (![]()
2010, March 8), more
efficient than the traditional
TEMPO, were used to effect selective catalytic
oxidation.