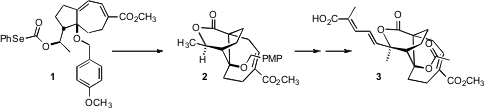
(-)-Pseudolaric Acid B (3), isolated from the bark of the golden larch
Pseudolarix kaempferi, shows potent antifungal activity. A key step in the total
synthesis of 3 described
(J. Am. Chem. Boc-(S)-3-Amino-3-phenylpropanal Price Soc. 2008, 130, 16424.
DOI: 10.1021/ja806724x)
by Barry M. Trost of Stanford University was the free radical cyclization
of 1 that established the angular ester and the trans ring fusion of 2
and thus of 3. PMID:34235739
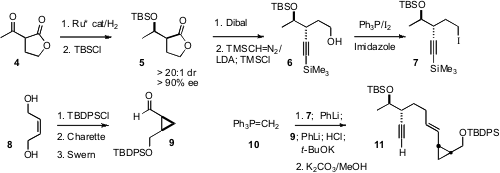
To prepare the bicyclic skeleton of 1, the authors envisioned the
Rh-mediated intramolecular addition of the alkyne of 11 to the alkenyl
cyclopropane. The acyclic centers of 11 were established by Noyori
hydrogenation of (equilibrating) racemic 4. One enantiomer reduced much
more quickly than did the other, leading to 5. 2231744-57-1 In stock The absolute configuration
of the cyclopropane was set by Charette cyclopropanation of the monosilyl ether
of the inexpensive diol 8. The two components were then coupled using a
Corey-Schlosser protocol. Alkylation of the ylide 10 with 7 gave a
new phosphonium salt, that in situ was deprotonated and condensed with the
aldehyde 9. The resulting betaine was deprotonated and quenched, then
exposed again to base to give the trans alkene 11. It is important in
this procedure to use PhLi as the base, since the alkyl lithium can displace the
alkyl group on phosphorus.
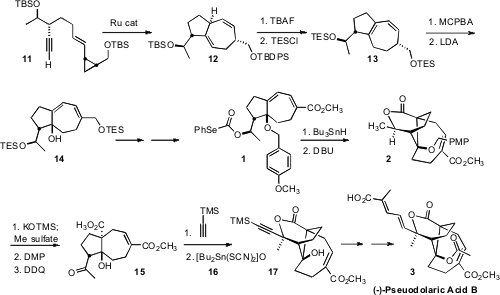
The product from Ru-catalyzed cyclization was the expected 1,4-diene 12.
Fortunately, it was found that TBAF desilylation led to concomitant alkene
migration, to give the more stable conjugated diene 13. Selective
epoxidation of the more electron-rich alkene followed by exposure to strong base
then delivered 14, with the requisite angular oxygenation established.
Pseudolaric Acid B (3) would be derived from cyclization of the
selenocarbonate of a tertiary alcohol. In fact, however, attempted cyclization
of such selenocarbonates led only to decarboxyation and reduction. Even with the
selenocarbonate 1 prepared from the secondary alcohol, the cyclization to
2 required careful optimization, including using not AIBN, but
azobis(dicyclohexylcarbonitrile) as the radical initiator.
Acetylide addition to the ketone 15 could be effected with high
diastereocontrol, but lactone construction proved elusive. Alkaline conditions
led quickly to addition of the angular hydroxyl to the activated alkene in the
seven-membered ring. Eventually it was found that the ester exchange catalyst
16 developed by Otera delivered 17, that could be carried on to (-)-Pseudolaric
Acid B (3).