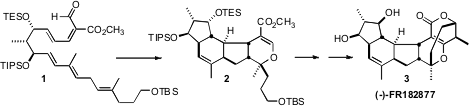
The Streptomyces metabolite (-)-FR182877 (3) binds to and stabilizes
microtubules, showing the same potency of anticancer activity as Taxol. Masahisa
Nakada of Waseda University assembled
(Angew. PMID:24635174 Chem. Int. Ed. Formula of 6-Bromo-2,7-naphthyridin-1(2H)-one 2009, 48, 2580.
DOI: 10.1002/anie.200900097)
the hexacyclic ring system of 3 by the tandem
intramolecular Diels-Alder – intramolecular hetero
Diels-Alder cyclization of
1, generating seven new stereogenic centers in a single step. (5-Bromopyrazin-2-yl)methanol Formula
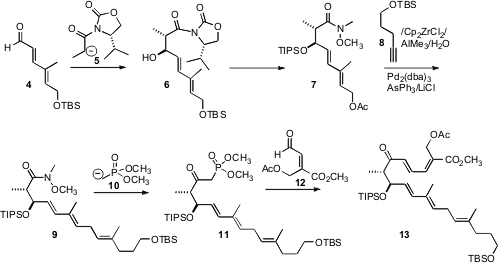
The construction of the pentaene substrate 1 started with the known
aldehyde 4, prepared by homologation of commercial ethyl
3-methyl-4-oxocrotonate. Addition of the propionyl oxazolidine anion 5
proceeded with high diastereocontrol, to give 6. The acyl oxazolidinone
was not an efficient acylating agent, so it was converted to the Weinreb amide.
Protection and deprotection then delivered the allylic acetate 7.
The key step in the pentaene assembly was the carefully optimized
Negishi-Wipf methylation of 8, followed by Pd-mediated coupling of the
alkenyl organometallic so generated with the allylic acetate, to give 9.
Condensation of the derived keto phosphonate 11 with the known aldehyde
12 then delivered the enone 13.
The Nakada group has worked extensively on the intramolecular Diels-Alder
reaction of substrates such as 1. They have shown that protected anti
diols such as 1 cyclize with substantial diastereocontrol and in the
desired sense. In contrast, cyclizations of protected syn diols proceed with
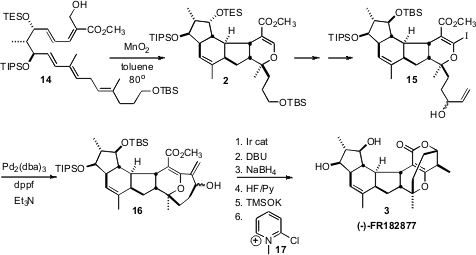
poor diastereocontrol. The enone 13 was therefore reduced to the anti
diol and protected, leading to 14. Oxidation of 14 at room
temperature led to a complex mixture, but slow oxidation at elevated temperature
delivered 2. Although the yield of 2 was not much better than if
the reactions were carried out sequentially, first the intramolecular
Diels-Alder cyclization, then the intramolecular hetero Diels-Alder cyclization,
with the cascade protocol pure 2 was more readily separated from the
reaction matrix.
With 2 in hand, there was still the challenge of assembling the
seven-membered ring. Cyclization was effected with an intramolecular
Heck
protocol. The two diastereomers of the allylic alcohol 15 cyclized with
comparable efficiency. Ir-catalyzed alkene migration then converted the allylic
alcohols to a mixture of ketones, that was equilibrated to give the more stable
diasteromer. Reduction of the ketone then set the last stereogenic center of
3. Deprotection and subsequent lactone formation completed the synthesis of
(-)-FR182877 (3).