Enantioselective hydrogenation of enamides is a well-established transformation.
The corresponding reduction of enamines has been elusive. Qi-Lin Zhou of Nankai University designed
(J. Am. Chem. Soc. 2009, 131, 1366.
DOI: 10.1021/ja808358r)
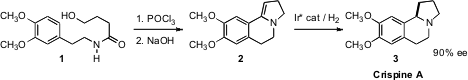
an Ir catalyst that reduced 2 to the Carpus alkaloid
Crispine A (3) in high ee.
Direct conversion of C-H to C-C bonds is a powerful synthetic transformation.
Liming Zhang, now at the University of California, Santa Barbara, observed
(J. Am. 1338257-80-9 manufacturer Chem. Price of Methyl 6-(chloromethyl)picolinate Soc. 2009, 131, 8394.
DOI: 10.1021/ja903531g)
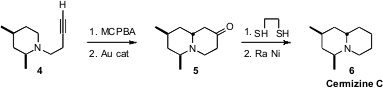
that a gold catalyst converted
the N-oxide of 4 into 5, that was then deoxygenated to give
Cermizine C (6). PMID:24487575 The gold catalyst and the N-oxide combined to convert
the alkyne into an α-keto carbene, in the process reducing the N-oxide back to
the amine. The carbene then abstracted a hydride from the carbon adjacent to the
amine, generating an intermediate that collapsed to give 5 with high
diastereocontrol.
Tangutorine (10), isolated from the leaves of Nitraria tangutorum, affects the
morphology of human colon cancer cells. In a biomimetic approach, Erwan Poupon
of the Université Paris-Sud stirred
(Org. Lett. 2009, 11, 1891.
DOI: 10.1021/ol9002916)
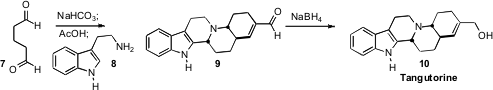
glutaraldehyde (7) with bicarbonate to give an unstable carbocyclic dimer.
Addition of tryptamine in acetic acid delivered the pentacyclic product 9,
that was reduced with borohydride to give the crystalline Tangutorine (10).
FR901483, a potent immunosuppressive isolated from a Cladobotyrum
fermentation broth, presents an challenging array of stereogenic centers in its
tricyclic skeleton. Michael A. Kerr of the University of Western Ontario prepared
(Org. Lett. 2009, 11, 777.
DOI: 10.1021/ol802870c)
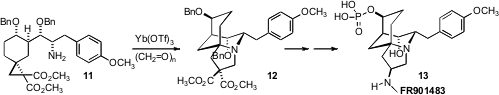
the activated cyclopropane 11, then effected intramolecular dipolar
opening with an intermediate imine, yielding the tricyclic 12.
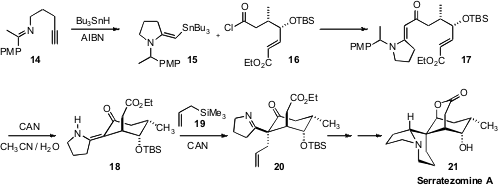
The Lycopodium alkaloid Serratezomine A (21) presents a similarly challenging
array of stereogenic centers in its tetracyclic structure. Jeffrey N. Johnston
of Vanderbilt University constructed
(J. Am. Chem. Soc. 2009, 131, 3470.
DOI: 10.1021/ja900536d)
the pyrrolidine ring of 15 using the imine free radical acceptor that he had
previously developed. Having the alkene-Sn bond in place then enabled coupling
with the acid chloride 16. Oxidative deprotection of 17 freed the enamine, that
added in a conjugate sense to the unsaturated ester, kinetically setting the
axial branch of 18. A second CAN-mediated step, allylation with 19, set the
quaternary center of Serratezomine A (21).