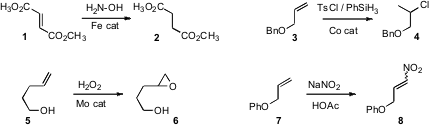
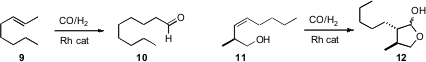Fabio Doctorovich of the Universidad de Buenos Aires reported(J. Org. 201929-84-2 Chemscene Chem. 2008, 73, 5379DOI: 10.1021/jo800302v)that hydroxylamine in the presence of an Fe catalyst reduced alkenes such as 1,but not ketones or esters. Erick Carreira of ETH Zürich developed(Angew. Chem. Int. Ed. 2008, 47, 5758DOI: 10.1002/anie.200801760)mild conditions for the hydrochlorination of mono-, di- and trisubstituted alkenes. Ramgopal Bhattacharyya of Jadavpur University established(Tetrahedron Lett. 2008, 49, 6205DOI: 10.1016/j.tetlet.2008.08.028)a simple Mo-catalyzed protocol for alkene epoxidation.
Nitro alkenes are of increasing importance as acceptors for enantioselective organocatalyzedcarbon-carbon bond formation. Matthias Beller of the Universität Rostock found(Adv. 6-Aminobenzo[c][1,2]oxaborol-1(3H)-ol Purity Synth. Catal. 2008, 350, 2493DOI: 10.1002/adsc.200800509)that an alkene such as 7 was readily converted to the corresponding nitroalkene8 by exposure to of NO gas. The reaction could also be effected with NaNO2/HOAC.
Two complementary protocols for Rh-catalyzed alkene hydroformylation have been reported. Xumu Zhang of Rutgers University devised(Org. PMID:23996047 Lett. 2008, 10, 3469DOI: 10.1021/ol801247v)a ligand system that cleanly migrated the alkene of9, then terminally hydroformylated the resulting monosubstituted alkene, to give 10. Kian L. Tan of Boston College designed(J. Am. Chem. Soc. 2008, 130, 9210DOI: 10.1021/ja803011d)a ligand such that the hydroformylation of the internal alkene of 11was directed to the end of the alkene proximal to the directing OH, delivering 12.
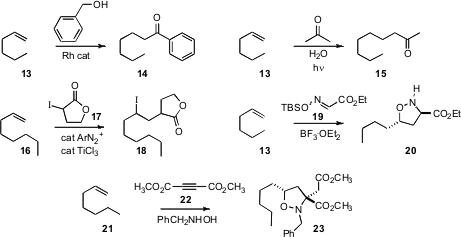
Several other methods for the functionalizing homologation of alkenes have been put forward. Chul-Ho Jun of Yonsei University assembled(J. Org. Chem. 2008, 73, 5598DOI: 10.1021/jo800862q)a Rh catalyst that effected the oxidative acylation of a terminal alkene 13 with a primary benzylic alcohol, to give the ketone 14. For now, this approach is limited to less expensive alkenes, as the alkene, used in excess, is the reductant in the reaction. The other procedures outlined here require only stoichiometric alkene. Yasuhiro Shiraishi of Osaka University devised(Org. Lett. 2008, 10, 3117DOI: 10.1021/ol800999s)a simple photoprocess for adding acetone to a terminal alkene 13 to give the methyl ketone 14, in what is presumably a free radical reaction. Chaozhong Li of the Shanghai Institute of Organic Chemistry found(Tetrahedron Lett. 2008, 49, 7380DOI: 10.1016/j.tetlet.2008.10.070)that reduction of a benzenediazonium salt with aqueous TiCl3 generated an effective catalyst for the free radical atom transfer addition of α-iodoesters and α-iodonitriles to alkenes such as 17, to give the adduct 18.
Dipolar cycloaddition can also be used to homologate alkenes. Osamu Tamura of Showa Pharmaceutical University found(J. Org. Chem. 2008, 73, 7164DOI: 10.1021/jo800878p)that exposure of 19 to BF3.OEt2 generated a species that addedto the alkene 13 with high diastereocontrol, to give 20.Gilles Dujardin of the Université du Maine combined(Org. Lett. 2008, 10, 4493DOI: 10.1021/ol8017243)the acetylenic ester 22 with N-benzylhydroxylamine to give a dipole, that added to the alkene 21 to give 23, establishing a quaternary center with high diastereocontrol.
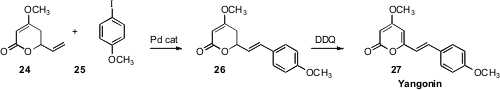
The Heck reaction has mostly been used with alkenes bearing electron-withdrawing groups, but in fact the intermediate aryl organopalladium species can insert into a wide variety of alkenes. Michèle David of the Université de Rennes1 took advantage(Tetrahedron Lett. 2008, 49, 6607DOI: 10.1016/j.tetlet.2008.07.102)of this, adding the iodide 25 to the alkene 24, to give 26. DDQ oxidation of 26 gave the lactone Yangonin 27, derived from the kava-kava root.