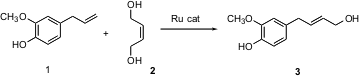
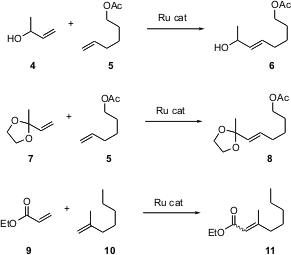
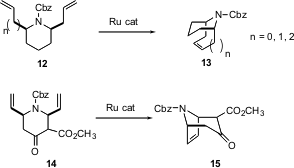
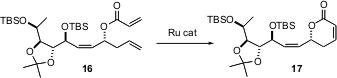Alkene metathesis (e.g. 1 + 2 -> 3) has been known at least since the 1950’s. Until Robert Grubbs of Caltech developed stable and versatile Ru catalysts for this transformation, however, this reaction was little used.
Professor Grubbs recently published (J. Am. Chem. Soc. 2003, 125, 10103.DOI: 10.1021/ja027472t / J. PMID:23983589 Am. Methyl aminolevulinate (hydrochloride) custom synthesis Chem. Soc. 2003, 125, 11360.DOI: 10.1021/ja0214882)two detailed articles on activation and selectivity in this reaction. Buy5-Bromo-1H-imidazole-2-carboxylic acid The first article addresses variations on catalyst design. The second paper defines several types of alkenes, and lays out rules that allow one to predict which pairs of alkenes will dimerize efficiently. While it is not possible to summarize all of their results in this limited space, some highlights include:
It is also not possible to even partially review all the many applications in synthesis that have already been demonstrated for the Grubbs reaction. I have chosen to focus on three papers. For those desiring to deploy the Grubbs catalyst only from time to time, storage and handling become serious issues. We have found (J. Org. Chem. 2003, 68, 6047.DOI: 10.1021/jo030005p)that the commercial catalyst dissolved in paraffin wax can be stored exposed to the laboratory atmosphere for many months and still retain full activity. The example above of 1 + 2 -> 3 is taken from that paper.
Steve Martin of UT Austin has reported (J. Org. Chem. 2003, 68, 8867.DOI: 10.1021/jo0349936)a detailed study of the synthesis of bridged azabicyclic structures via ring-closing alkene metathesis. Some examples of his work include the conversion of 12to 13, efficiently forming six, seven and eight membered rings. He also demonstrated five-membered ring formation with the conversion of 14 to 15, which has the cocaine skeleton.
A real concern when attempting the Grubbs reaction with a complex substrate is the stability of other alkenes. J. Alberto Marco of the University of Valencia, Spain has shown (J. Org. Chem.,2003, 68, 5672.DOI: 10.1021/jo034470y)that 16 is converted to 17 without isomerization of the Zalkene. Less congested alkenes might not be so resistant.