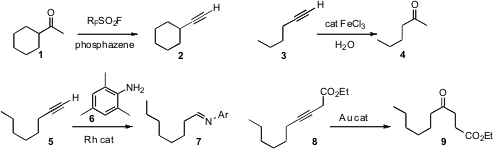
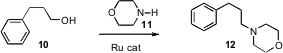Ilya M. Lyapkalo of the Academy of Sciences of the Czech Republic, Prague, showed(Synlett 2009, 558.DOI: 10.1055/s-0028-1087919)that a ketone 1 reacted with the inexpensive nonafluorobutanesulfonyl fluoridein the presence of a phosphazene base to give first the enol sulfonate, and then thealkyne 2. The method worked well for aldehydes also.
Christophe Darcel of the Université de Rennes I developed(Adv. Synth. Catal. 2009, 351, 367.DOI: 10.1002/adsc.200800666)an inexpensive Fe catalyst for the hydration of a terminal alkyne 3 to the ketone 4. Carlos Alonso-Moreno and Antonio Otero of the Universidad de Castilla-La Mancha devised (Adv. Synth. Catal. 2009, 351, 881.DOI: 10.1002/adsc.200800786)a Rh catalyst for the complementary hydration of a terminal alkyne 5 to thealdehyde, by way of the imine 7. Internal alkynes often give mixtures of ketoneson hydration, but Bo Xu and Gerald B. Hammond of the University of Louisville found (J. Org. Chem. PMID:24818938 2009, 74, 1640.DOI: 10.1021/jo802450n)a gold catalyst that converted an alkynyl ester 8 into theγ-keto ester 9.
Jonathan M. J. Ruthenium(III) acetate uses Williams of the University of Bath developed(J. Am. (3-Bromo-1-propyn-1-yl)cyclopropane Chemscene Chem. Soc. 2009, 131, 1766,DOI: 10.1021/ja807323a; Tetrahedron Lett. 2009, 50, 3374,DOI: 10.1016/j.tetlet.2009.02.129)a Ru-catalyzed protocol for the alkylation of an amine 11 with an alcohol10. The reaction proceeded by oxidation of the alcohol to the aldehyde,imine formation, and reduction using the hydride generated by the initial oxidation.José Luis García Ruano of the Universidad Autónoma de Madrid uncovered(Chem. Commun. 2009, 404.DOI: 10.1039/b816846f)a similar conversion mediated by Raney Ni.
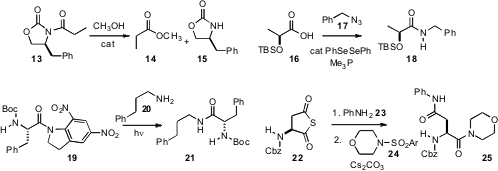
There has been a great deal of work recently on the preparation and reaction of amides. Susumu Saito of Nagoya University prepared(J. Am. Chem. Soc. 2009, 131, 8748.DOI: 10.1021/ja9029494)a diaryl boronic acid that catalyzed the methanolysis of an imide 13 to the methyl ester14 and the oxazolidinone 15. Jaume Vilarrasa of the Universitat de Barcelona reported(J. Org. Chem. 2009, 74, 2203.DOI: 10.1021/jo802825e)the catalyzed condensation of an acid 16 with an azide 17 to give the amide18. Both aryl and aliphatic azides participated in the reaction, and the enantiomericintegrity of the amide was maintained. Christian G. Bochet of the University of Fribourg developed(J. Org. Chem. 2009, 74, 4519.DOI: 10.1021/jo900442p)the photolabile amide 19. Long wave UV radiation, that did not disturb other photoactivatable protecting groups, then effected acylation of 20 to give 21, with maintenance of the enantiomeric integrity of 19.
David Crich, now at CNRS de Gif-sur-Yvette, found(J. Org. Chem. 2009, 74, 3886.DOI: 10.1021/jo900532e)that the enantiomerically-pure thioanhydride 22 could be coupled sequentially first with an amine 23 and then with an arenesulfonamide 24 to give the unsymmetrical diamide 25. This approach was used to prepare diastereomerically-pure glycodipeptides.
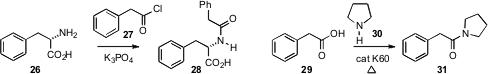
Li Zhang of Boehringer Ingelheim took(Tetrahedron Lett. 2009, 50, 2964.DOI: 10.1016/j.tetlet.2009.03.220)a simpler approach to amide construction, demonstrating that K3PO4 mediated the coupling of 26 and 27 to give 28 with minimal racemization.James H. Clark of the University of York established(Chem. Commun. 2009, 2562.DOI: 10.1039/b901581g)an even milder protocol, heating (toluene, reflux) the acid29 with the amine 30 in the presence of 10weight percent of calcined Kieselgel 60 to give the amide 31.