A
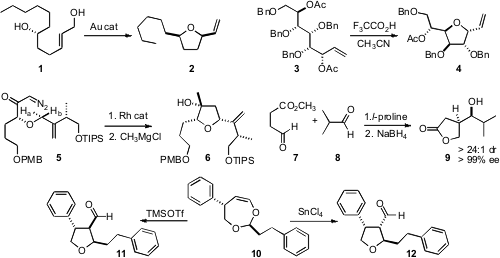
Since five-membered ring ethers often do not show good selectivity on
equilibration, single diastereomers are best formed under kinetic control. Aaron
Aponick of the University of Florida demonstrated
(Org. Lett. 2008, 10, 669.
DOI: 10.1021/ol703002p)
that under gold catalysis, the allylic alcohol 1 cyclized
to 2 with remarkable diastereocontrol. Buy1450754-37-6 Morpholin-2-one Purity Six-membered rings also formed
with high cis stereocontrol. Ian Cumpstey of Stockholm University showed
(Chem. Commun. 2008, 1246.
DOI: 10.1039/b718060h)
that with protic acid, allylic acetates such as
3 cyclized with clean inversion at the allylic center, and concomitant
debenzylation. PMID:24733396 J. Stephen Clark of the University of Glasgow found
(J. Org. Chem. 2008, 73, 1040.
DOI: 10.1021/jo702111u)
that Rh catalyzed cyclization of 5 proceeded with high selectivity for insertion into Ha, leading to the alcohol
6. Saumen Hajra of the Indian Institute of Technology, Kharagpur took
advantage (J. Org. Chem. 2008, 73, 3935.
DOI: 10.1021/jo8005733)
of the reactivity of the aldehyde of 7, effecting selective addition of 7 to 8,
to deliver, after reduction, the lactone 9. Tomislav Rovis of Colorado State University observed
(J. Org. Chem. 2008, 73, 612.
DOI: 10.1021/jo702071v)
that 10 could be cyclized selectively to either 11 or 12.
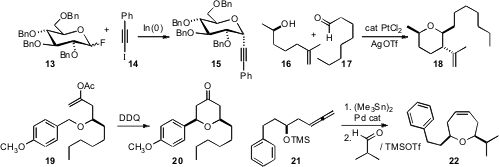
Nadège Lubin-Germain, Jacques Uziel and Jacques Augé of the University of Cergy-Pontoise devised
(Org. Lett. 2008, 10, 725.
DOI: 10.1021/ol7029257)
conditions for the indium-mediated coupling of glycosyl fluorides such as 13
with iodoalkynes such as 14 to give the axial C-glycoside 15.
Katsukiyo Miura and Akira Hosomi of the University of Tsukuba employed
(Chemistry Lett. 2008, 37, 270.
DOI: 10.1246/cl.2008.270)
Pt catalysis to effect in situ equilibration of the alkene 16 to the more stable regioisomer. Subsequent
condensation with the aldehyde 17 led via Prins cyclization to the ether
18.
Paul E. Floreancig of the University of Pittsburgh showed
(Angew. Chem. Int. Ed. 2008, 47, 4184.
DOI: 10.1002/anie.200706002) that
Prins cyclization could be also
be initiated by oxidation of the benzyl ether 19 to the corresponding
carbocation. Chan-Mo Yu of Sungkyunkwan University developed
(Org. Lett. 2008, 10, 265.
DOI: 10.1021/ol7026569)
a stereocontrolled route to seven-membered ring
ethers, by Pd-mediated stannylation of allenes such as 21, followed by
condensation with an aldehyde.
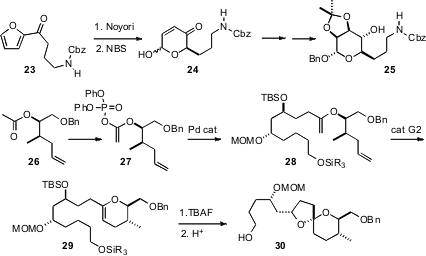
George A. O’Doherty of West Virginia University devised
(J. Org. Chem. 2008, 73, 1935.
DOI: 10.1021/jo800691v)
an alternative route toward C-glycosides, by enantioselective reduction of the furyl ketone
23 followed by oxidative
rearrangement. Haruhiko Fuwa and Makato Sasaki of Tohoku University, en route to a synthesis of Attenol A, established
(Org. Lett. 2008, 10, 2549.
DOI: 10.1021/ol800815t)
a highly-convergent route to spiro ethers such as 30, based on
Pd-catalyzed coupling of enol phosphates such as 27, followed by
ring-closing metathesis and acid-catalyzed cyclization.