Ultrasound-Promoted Heck Reaction in Room Temperature Ionic Liquids
In 2001, Srinivasan and co-workers form National Chemical Laboratory, Pune,
described the first Heck reaction performed in
ionic liquids (IL) under
ultrasound irradiation. In their article, many aryl iodides were coupled with
activated alkenes with remarkable results in terms of yields, reaction time and
selectivity.
(Chem. Commun. BuyH2N-PEG2-CH2COOtBu 2001, 1544.
DOI: 10.1039/b104532f)
This new synthetic methodology is quite superior to conventional Heck reactions, which
are generally carried out in polar solvents such as DMF and NMP under reflux
conditions with long reaction times. In this reaction, the sound wave activation
was found to be essential because no conversion of the starting materials took
place when it was repeated under conventional stirring. Furthermore, in some
cases, conventional Heck alkenylations lead to the formation of mixtures of E/Z
diastereoisomers; nevertheless, the ultrasound-mediated protocol furnished
exclusively the E products. It is noteworthy that phenylacetylene also undergoes
the reaction; thus, this procedure can be used as an alternative to the
Sonogashira reaction. PMID:23398362 It is also important to mention that the
ultrasound-mediated Heck reaction does not need the use of phosphine ligands to
stabilize the Pd(0) catalyst. Indeed, the reaction of the Pd(II) salt with ionic
liquid leads to the formation of a Pd-carbene complex with
alkylimidazol-2-ylidene. This complex is then reduced sonochemically in situ by
a single electron transfer (SET) process, to afford a Pd(0) species that is the
active catalyst for the Heck reaction. Price of 5-Bromonicotinaldehyde
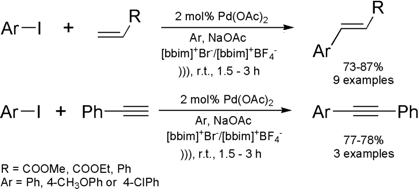
Ultrasound-Promoted Suzuki Reaction in Room Temperature Ionic Liquid
The same group of Indian researchers cited above
disclosed the first Suzuki reaction performed in ionic liquids with ultrasound
activation.
(Chem. Commun. 2002, 616.
DOI: 10.1039/b111271f)
The reactions between phenylboronic acid and aryl halides have been carried out employing [bbim]+[BF4]– / MeOH
as solvent system. In their work, the
Suzuki reaction was first
investigated employing Pd(OAc)2 as catalyst, in the absence of phosphine ligands.
Under these conditions a series of 11 biaryls were synthesized in good yields
and short reaction times. The reaction has presented a good scope of
applications, since aryl halides containing electron-donating groups (EDG)
reacted as well as the ones containing electron-withdrawing groups (EWG). It is
also important to note that even chlorobenzenes undergo the Suzuki reaction to
afford biaryls in 42-65% yields. Although yields for these latter conversions
were moderate, chlorobenzenes are known to have poor reactivity under Suzuki
conditions. Thus, this acoustic technique is quite valuable for coupling these
substrates with boronic acids. In the same paper, the authors improved the
reaction by employing tetrafluoroborate bis-butylimidazol-2-ylidene-palladium
(II) carbene complex A instead of Pd(OAc)2. This modification has allowed them to
recycle and re-use the palladium catalyst without losses in terms of yields and
reaction times.
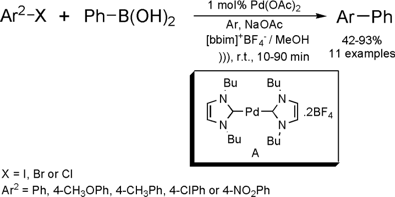
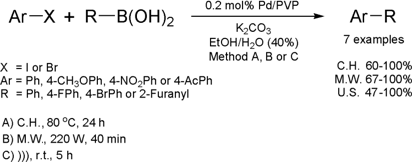
Ultrasound-Assisted Suzuki-Miyaura Cross-Coupling Reaction
Catalyzed by Pd/PVP
The group of Antunes from Federal University of Rio de
Janeiro has developed a new protocol for Suzuki cross coupling reaction
catalyzed by palladium nanoparticles stabilized in poly(vinylpyrrolidone) (PVP).
The new method for the Suzuki reaction was investigated under conventional or
microwave heating (MW) as well as ultrasound irradiation (US).
(Tetrahedron Lett. 2008, 49, 3895.
DOI: 10.1016/j.tetlet.2008.04.061)
The reaction presented a large range of applications, since aryl iodides or bromides containing EDG or EWG
reacted with arylboronic acids to furnish biaryls in good yields. Although the
best results have been achieved when using dielectric heating (MW), ultrasound
irradiation was also attractive when compared with conventional heating. In the
experiments performed under conventional heating, coupled products have been
obtained in good yields after 18 h of reaction time while using sound wave
activation; similar results have been obtained in just 5 h of reaction. In
addition, the catalyst could be recycled and re-used two times without loss in
yield.
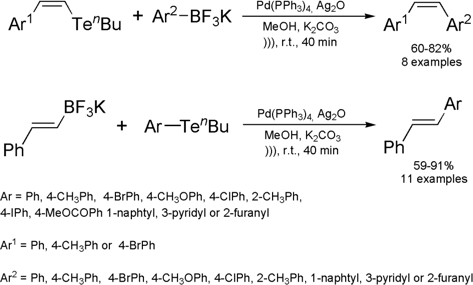
Ultrasound-Assisted cross-coupling reaction between potassium
trifluoroborate salts and organotellurides
Hélio A. Stafani and Rodrigo Cella from the University of São Paulo, Brazil, have reported a new method for
preparation of Z– or E-stilbenes by the ultrasound-assisted cross-coupling
reaction between potassium trifluoroborate salts and aryl- or vinyltellurides.
(Tetrahedron 2006, 62, 5656.
DOI: 10.1016/j.tet.2006.03.090)
The reaction has been employed
to synthesize a series of 22 alkenes with defined stereochemistry, giving good
yields in 40 min. For comparison proposes, the reaction was carried out under
magnetic stirring and it was observed that much of the starting material
remained unchanged after 24 h of reaction. After repeating the same reaction
under reflux conditions for 18 h the coupled product was obtained in 63% yield.
Thus, it is clear that the use of sound wave activation is not just helpful but
essential for performing this coupling reaction. Additionally, it is also worth
noting that the reaction presented good utility and even tolerated ester groups,
which are sensitive to basic conditions. Later, the same research group has used
this protocol with some modifications for the synthesis of 1,3-dienes
(Tetrahedron Lett. 2006, 47, 5075.
DOI: 10.1016/j.tetlet.2006.05.088)
1,3-enynes (Synlett 2008, 1889.
DOI: 10.1055/s-2008-1078507)
(Z)-(2-chlorovinyl)alkenes (Tetrahedron Lett. 2008, 49, 4713.
DOI: 10.1016/j.tetlet.2008.05.129)
and symmetrical biaryls (Synlett 2008 2321.
DOI: 10.1055/s-0028-1087244)
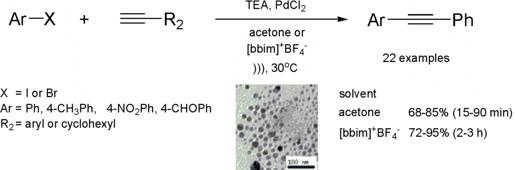
The Sonogashira reaction
catalyzed by Copper- and Ligand-free Pd(0) Nanoparticles under Ultrasound
Irradiation
Indian researchers disclosed in 2005 the first ultrasound-mediated
Pd(0) nanoparticle-catalyzed cross-coupling reaction between aryl iodides or
bromides and terminal acetylenes (Sonogashira reaction)
(J. Org. Chem. 2005, 70, 4869.
DOI: 10.1021/jo0503815).
It was found that this reaction worked well with a large number of substrates
in the absence of any further phosphine ligand or
copper co-catalyst. The reaction was examined in acetone as well as in IL
[bbim]BF4 under basic conditions. It was found that use of the molecular solvent
furnished the desired coupled products in a substantially shorter time than when
using IL, but in slight lower yields. Although, the use of IL requires more time
for complete reaction, it allowed the recycling and reuse of the catalyst with
little loss of activity after five runs. In order to learn the importance of
ultrasound irradiation on the reaction, it was repeated under silent conditions
(without ultrasound) and in this case no change took place even after several
hours of stirring. Besides the improvement in terms of reaction time and yields,
it is important to mention that no homocoupling between terminal alkynes (Glaser
coupling) was found in the reactions employing aryl iodides, while homocoupled
products were present to the extent of just 6-7% for aryl bromides.
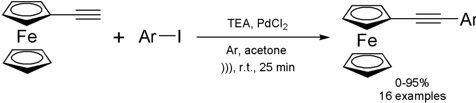
Synthesis of 1-ferrocenyl-2-arylacetylenes under ultrasound irradiation
Although most of
ultrasound-mediated cross-coupling reactions are geared towards functionalizing
organic compounds, they are also applicable to substrates containing an
inorganic moiety. The group of Baohua Chen from Lanzhou University, China,
reported the palladium-catalyzed coupling reaction of ethynylferrocene with aryl
iodides (Sonogashira coupling) under ultrasound irradiation
(Catal. Comm. 2008, 9, 976.
DOI: 10.1016/j.catcom.2007.09.039).
The protocol afforded the desired
products in 0-95% yields in 25-35 min of reaction. Besides the good yields and
shorter reaction times, the main advantage of this method is the use of small
loadings of cheap, commercially available PdCl2 as catalyst. The scope of the
reaction is quite broad, but electron-poor aryl iodides were generally
superior to electron-rich ones