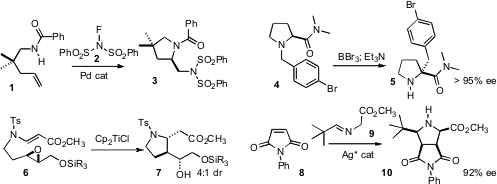
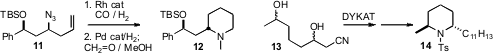Forrest E. Michael of the University of Washington described
(Org. Lett. 5,6-Dichloropyridazin-3(2H)-one In stock Sodium triacetoxyborohydride In stock 2009, 11, 1147.
DOI: 10.1021/ol9000087)
the Pd-catalyzed aminative cyclization of 1 to the differentially-protected
diamine 3. Peter Somfai of KTH Chemical Science and Engineering observed
(Org. Lett. 2009, 11, 919.
DOI: 10.1021/ol8028803)
that [1,2]-rearrangement of 4 proceeded to deliver 5 with near-perfect
maintenance of enantiomeric excess. PMID:23514335 Tushar Kanti Chakraborty of the Central Drug
Research Institute, Lucknow applied
(Tetrahedron Lett. 2009, 50, 3306.
DOI: 10.1016/j.tetlet.2009.02.063)
the Ti(III) reduction of epoxides to the
Sharpless-derived ether 6,
leading to the pyrrolidine 7. Chun-Jiang Wang of Wuhan University devised
(Chem. Commun. 2009, 2905.
DOI: 10.1039/b902556a)
a silver catalyst that directed the absolute sense of the
dipolar addition
of 9 to 8 to give 10.
Homoallyic azides such as 11 are readily prepared in high enantiomeric
excess from the corresponding alcohol. Bernhard Breit of Albert-Ludwigs-Universität,
Freiburg and André Mann of the Faculté de Pharmacie, Illkirch showed
(Org. Lett. 2009, 11, 261.
DOI: 10.1021/ol802314g)
that Rh-mediated hydroformylation could be effected in the presence of the
azide. Subsequent reduction delivered the
piperidine 12. Jan-E. Bäckvall
of Stockholm University applied
(J. Org. Chem. 2009, 74, 1988.
DOI: 10.1021/jo8025109)
the protocol for dynamic kinetic asymmetric transformation (DYKAT) that he had
developed to the cyanodiol 13. Remarkably, a single enantiomerically-pure
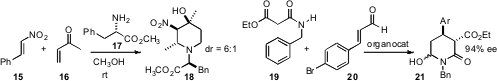
diasteromer emerged, which he carried on to 14. Xiaodong Shi of West
Virginia University found
(Org. Lett. 2009, 11, 2333.
DOI: 10.1021/ol900708d)
that the stereogenic center of 17, even though it ended up outside the ring,
directed the absolute configuration of the other centers of 18 as they
formed. Jan Vesely of Charles University and Albert Moyano and Ramon Rios of the
Universitat de Barcelona established
(Tetrahedron Lett. 2009, 50, 1943.
DOI: 10.1016/j.tetlet.2009.02.049)
that an organocatayst directed the absolute configuration in the
addition of 19 to 20 to give 21.
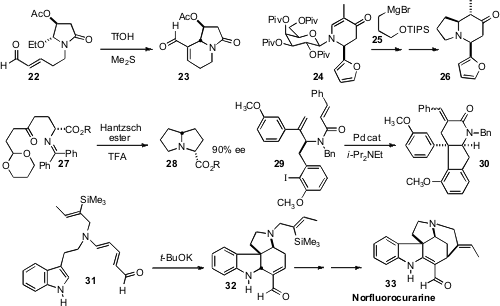
Osamu Tamura of Showa Pharmaceutical University effected
(Org. Lett. 2009, 11, 1179.
DOI: 10.1021/ol900032h)
cyclization of the malic acid-derived amide 22
to give 23 with high diastereocontrol. Horst Kunz of Johannes
Gutenberg-Universität Mainz demonstrated
(Angew. Chem. Int. Ed. 2009, 48, 2228.
DOI: 10.1002/anie.200805606)
that the galactosylamine-derived auxiliary of 24
directed conjugate addition first to prepare 24, then again in the
conversion of 24 to 26. Keiji Maruoka of Kyoto University used
(Org. Lett. 2009, 11, 2027.
DOI: 10.1021/ol900477x)
an organocatalyst-directed conjugate addition to prepare 27, that he then
carried on to 28. Hiroyuki Ishibashi of Kanazawa University established
(J. Org. Chem. 2009, 74, 2624.
DOI: 10.1021/jo802787j)
conditions for the cascade cyclization of 29 to 30.
Diels-Alder cyclization of a triene such as 31 would offer rapid
access to polycyclic indole alkaloids. Christopher D. Vanderwal of the
University of California, Irvine developed
(J. Am. Chem. Soc. 2009, 131, 3472.
DOI: 10.1021/ja900640v)
alkaline conditions for this powerful transformation. He then
carried 32 on to the Strychnos alkaloid Norfluorocurarine (33).