Dactylolide (Jennings), Cytotrienin A (Hayashi), Lepadin B (Charette),
Blumiolide C (Altmann)
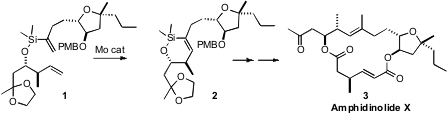
To assemble the framework of the cytotoxic macrolide Amphidinolide X (3),
Fèlix Urpí and Jaume Vilarrasa of the Universitat de Barcelona devised
(Org. Lett. 2008, 10, 5191.
DOI: 10.1021/ol8021676)
the ring-closing metathesis of the alkenyl silane 1. No Ru catalyst was effective,
but the Schrock Mo catalyst worked well. 2,3,4,5,6-Pentafluoroaniline Chemscene
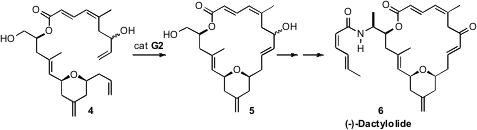
In the course of a synthesis of (-)-Dactylolide (6), Michael P.
Jennings of the University of Alabama offered
(J. Org. Chem. PMID:32926338 Price of 2-(1H-Pyrazol-3-yl)propan-2-ol 2008, 73, 5965.
DOI: 10.1021/jo8009853)
a timely reminder of the particular reactivity of allylic
alcohols in ring-closing metathesis. The cyclization of 4 to 5
proceeded smoothly, but attempted ring closing of the corresponding bis silyl
ether failed.
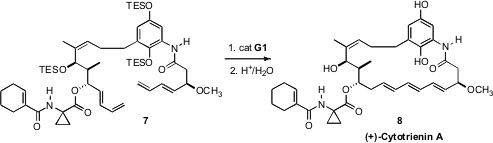
Polyenes such as (+)-Cytotrienin A (8) are notoriously unstable. It is
remarkable that Yujiro Hayashi of the Tokyo University of Science could
(Angew. Chem. Int. Ed. 2008, 47, 6657.
DOI: 10.1002/anie.200802079)
assemble the triene of 8
by the ring-closing metathesis of the highly functionalized precursor 7.
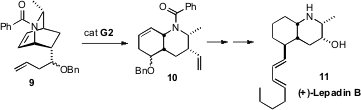
Bicyclo [2.2.2] structures such as 9 are readily available by the
addition of, in this case, methyl acrylate to an enantiomerically-pure
2-methylated dihydropyridine. André B. Charette of the Université de Montréal found
(J. Am. Chem. Soc. 2008, 130, 13873.
DOI: 10.1021/ja8068215)
that 9 responded well to
ring-opening/ring-closing metathesis, to give the
octahydroquinoline 10. Functional group manipulation converted 10
into the Clavelina alkaloid (+)-Lepadin B (11).
The construction of trisubstituted alkenes by ring-closing metathesis can be
difficult, and medium rings with their transannular strain are notoriously
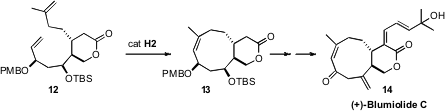
challenging to form. Nevertheless, Karl-Heinz Altmann of the ETH Zürich was able
(Angew. Chem. Int. Ed. 2008, 47, 10081.
DOI: 10.1002/anie.200804004), using the H2
catalyst, to cyclize 12 to cyclononene 13, the precursor to the
Xenia lactone (+)-Blumiolide C (14).
It is noteworthy that these five syntheses used four different metathesis
catalysts in the key alkene forming step. For the cyclization of 7, the
use of the Grubbs first generation catalyst G1, that couples terminal
alkenes but tends not to interact with internal alkenes, was probably critical
to success. The Hoveyda catalyst H2 is more expensive than the Grubbs
second generation catalyst G2, but can often be effective in applications
in which G2 is sluggish. The Schrock Mo catalyst is less user friendly
than the (relatively) air and moisture stable Ru catalysts, but is very reactive.