F
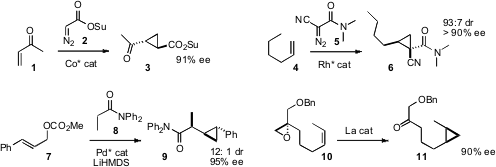
X. Peter Zhang of the University of South Florida extended
(Org. PMID:24507727 Lett. 2009, 11, 2273.
DOI: 10.1021/ol9005882)
Co-catalyzed asymmetric
cyclopropanation to the activated ester 2. The product 3 readily coupled with amines.
André B. 2-Amino-3-iodopyridine Order Charette of the Université de Montréal showed
(J. Am. Chem. Soc. 2009, 131, 6970.
DOI: 10.1021/ja902205f)
that even α-olefins such as 4 could be cyclopropanated in high ee with the
diazo amide 5. Xue-Long Hou of the Shanghai Institute of Organic Chemistry established
(J. Am. Chem. 3-Cyano-2-phenylpropanoic acid supplier Soc. 2009, 131, 8734.
DOI: 10.1021/ja902410w)
conditions for the enantioselective coupling of 7 and 8 to give 9, in
which sidechain chirality was also controlled. Tristan H. Lambert of Columbia University found
(J. Am. Chem. Soc. 2009, 131, 7536.
DOI: 10.1021/ja902137z)
that “methylene” could be transferred in an
intramolecular sense from the epoxide of 10 to the alkene, delivering the
cyclopropane 11 in high ee.
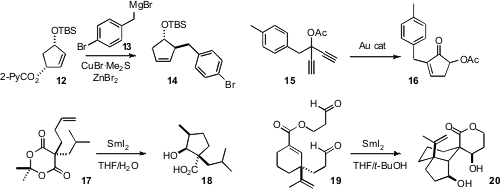
Yuichi Kobayashi of the Tokyo Institute of Technology established
(Org. Lett. 2009, 11, 1103.
DOI: 10.1021/ol802949c)
that the 2-picolinoxy leaving group worked well for the SN2′
coupling with 13 to give 14. Chang Ho Oh of Hanyang University developed
(J. Org. Chem. 2009, 74, 370.
DOI: 10.1021/jo802103g)
a new route to cyclopentenones such as 16, by gold-catalyzed cyclization of
diynes such as 15. David J. Procter of the University of Manchester used
(J. Am. Chem. Soc. 2009, 131, 7214,
DOI: 10.1021/ja901715d;
Tetrahedron Lett. 2009, 50, 3224,
DOI: 10.1016/j.tetlet.2009.02.025)
SmI2 to cyclize 17 to
18 and 19 to 20, each with high
diastereocontrol.
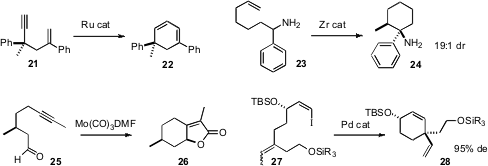
Yoshiaki Nishibayashi of the University of Tokyo devised
(Angew. Chem. Int. Ed. 2009, 48, 2534.
DOI: 10.1002/anie.200900392
Ru catalysts for the cyclization of an enyne such as
21 to the cyclohexadiene 22. Laurel L. Schafer of the University of British Columbia
developed (J. Am. Chem. Soc. 2009, 131, 2116.
DOI: 10.1021/ja808862w
a Zr catalyst for the diastereocontrolled cyclization of amino alkenes such as 23.
Hongbin Zhai of the Shangahi Institute of Organic Chemistry showed
(J. Org. Chem. 2009, 74, 2592.
DOI: 10.1021/jo900045k)
that the Mo-mediated cyclization of 25 also proceeded with high diastereocontrol.
Even more impressive was the selectivity Kozo Shishido of the University of Tokushima demonstrated
(Tetrahedron Lett. 2009, 50, 1279.
DOI: 10.1016/j.tetlet.2008.12.105)
for the cyclization of 27.
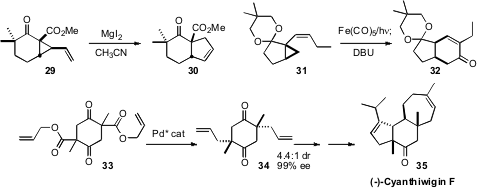
Professor Lambert established
(J. Am. Chem. Soc. 2009, 131, 2496.
DOI: 10.1021/ja809226x)
that the rearrangement of the vinyl cyclopropane 29 to the cyclopentene 30,
usually a high temperature process, could be effected with MgI2. We
(J. Org. Chem. 2009, 74, 2433.
DOI: 10.1021/jo802493k)
found that Fe-mediated cyclocarbonylation of 31 led to the cyclohexenone 32.
Brian M. Stoltz of Caltech prepared (Nature 2008, 453, 1228.
DOI: 10.1038/nature07046)
the diester 33 by dimerization of diallyl succinate followed by methylation. Pd-mediated
rearrangement delivered 34, the central ring of (-)-Cyanthiwigin (35), in high de
and ee..