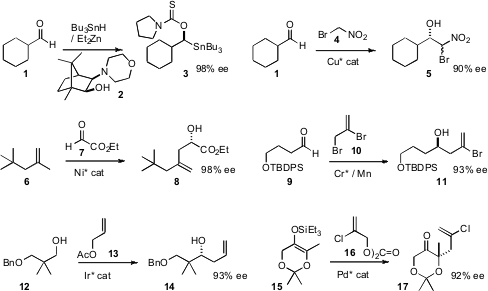
Enantiomerically-enriched alkoxy stannanes such as 3 are versatile intermediates for synthesis. John R. Falck of UT Southwestern found(Angew. Chem. Int. Ed. 2008, 47, 6586.DOI: 10.1002/anie.200802313)that the simple combination of Bu3SnH and Et2Zn generated areagent that added to aldehydes such as 1 under catalysis by the MIB aminoalcohol introduced by Nugent(Chem. Commun. 1999, 1369.DOI: 10.1039/a904042k)to give the adduct 3 in high ee. 3-(Trimethylsilyl)-2-propyn-1-ol Price Gonzalo Blay and José R. Pedro of the Universitat de València showed(Chem. Commun. 2008, 4840.DOI: 10.1039/b809739a)that it was possible to modulate the reactivity of the acidic 4, allowing catalyzed formationof the high ee adduct 5 to dominate. Xiaoming Feng of Sichuan University developed(J. Am. 1-Phenylbuta-2,3-dien-1-one web Chem. Soc. 2008, 130, 15770DOI: 10.1021/ja808023y)an Ni catalyst for the intermolecularene reaction of 6 with 7 to give8 in high ee.
Enantioselective allylation is a key transformation in current organic synthesis. PMID:24458656 Yoshito Kishi of Harvard University optimized(Org. Lett. 2008, 10, 3073.DOI: 10.1021/ol801093p)enantioselective Cr-mediated allylation, with a ligand that can be easilyrecovered and recycled. Michael J. Krische of UT Austin devised(J. Am. Chem. Soc. 2008, 130, 14891.DOI: 10.1021/ja805722e)a ligand-catalyst combination for effecting the enantioselective allylationof alcohols such as 12. Brian M. Stoltz of Caltech developed(Angew. Chem. Int. Ed. 2008, 47, 6873.DOI: 10.1002/anie.200801424)a protocol for the enantioselective allylation of the enol ether 15, leading to the construction of oxygenated quaternary centers. Adducts such as 11 and 17 are of interest, inter alia, as direct precursors, by elimination, of the corresponding alkynes.
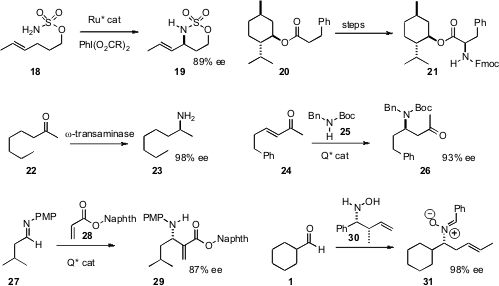
Simon Blakey of Emory University designed(Angew. Chem. Int. Ed. 2008, 47, 6825.DOI: 10.1002/anie.200801445)a Ru catalyst that mediated enantioselective intramolecular C-H amination, converting the simple alcohol derivative 18 into the versatile secondary amine 19 in high ee. We established(J. Org. Chem. 2008, 73, 9334.DOI: 10.1021/jo801781v)a procedure, based on diazo transfer followed by Rh-mediated intermolecular N-H insertion,for aminating menthyl esters and separating the product diastereomers. The menthyl group, easily removed (TFA) from 21, served as a useful reporter of ee, by 1H NMR of the upfield methyl doublets. Wolfgang Kroutil of the University of Graz found(Adv. Synth. Catal. 2008, 350, 2761.DOI: 10.1002/adsc.200800496)that ω-transaminases could effect the reductive amination of methyl ketones such as22 in high ee. In many cases, either enantiomer of the amine could be prepared, depending on the transaminase used.
Li Deng of Brandeis University developed (Angew. Chem. Int. Ed. 2008, 47, 7710.DOI: 10.1002/anie.200802785)a cinchona alkaloid derived catalyst for the enantioselective conjugate addition of 25 to enones. Manabu Node of Kyoto Pharmaceutical University reported(Org. Lett. 2008, 10, 2653.DOI: 10.1021/ol8007793)a related procedure for the addition of a recyclable enantiomerically-pure amine to α,β-unsaturated esters. Géraldine Masson and Jieping Zhu of CNRS Gif-sur-Yvette devised(J. Am. Chem. Soc. 2008, 130, 12596.DOI: 10.1021/ja805122j)a catalyst, also based on cinchona alkaloids, for the enantioselecitve azaMorita-Baylis-Hillman reaction, converting the imine 27 into 29 in substantial ee. Teck-Peng Loh of Nanyang Technological University designed(Org. Lett. 2008, 10, 2805.DOI: 10.1021/ol800925v)the enantiomerically- and diastereomerically-pure hydroxylamine 30. The initial adduct of 30 with the aldehyde 1 rearranged to deliver 31 in high ee and as a single geometric isomer.