(Cox), Salmochelin SX (Gagné), Botcinin F (Shiina), (-)-Saliniketal B
(Paterson), Haterumalide NA (Borhan)
Tohru Fukuyama of the University of Tokyo and Toshiyuki Kan of the University
of Shizuoka devised
(J. Am. 98642-15-0 manufacturer Chem. PMID:24982871 Soc. 117585-92-9 Chemical name 2008, 130, 16854
DOI: 10.1021/ja807676v)
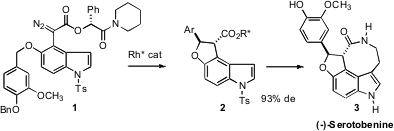
the chiral auxiliary-directed Rh-mediated cyclization of 1, setting the two stereogenic
centers of 2 with high stereocontrol. The ester 2 was carried on to the indole
alkaloid (-)-Serotobenine (3).
In the course of a synthesis of (-)-Aureonitol (6), Liam R. Cox of the
University of Birmingham developed
(J. Org. Chem. 2008, 73, 7616
DOI: 10.1021/jo801338t)
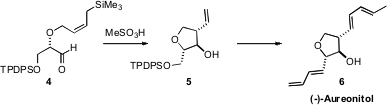
the diastereoselective intramolecular addition of an allyl silane 4 to give the
tetrahydrofuran 5. In analogy to what is known about the intramolecular
ene
reaction, the diastereocontrol observed for this cyclization may depend on the
allyl silane being Z.
Michel R. Gagné of the University of North Carolina found
(J. Am. Chem. Soc. 2008, 130, 12177
DOI: 10.1021/ja8041564)
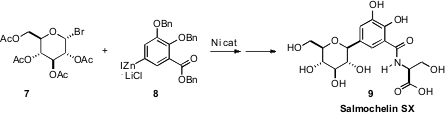
that the Ni-catalyzed coupling of organozinc halides could be
extended to glycosyl halides such as 7. This opened ready access to C-alkyl and
C-aryl glycosides, including Salmochelin SX 10.
Isamu Shiina of the Tokyo University of Science established
(Org. Lett. 2008, 10, 3153
DOI: 10.1021/ol801066y)
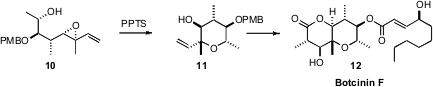
that the acid-mediated cyclization of the
Sharpless-derived epoxide 10 proceeded with clean inversion, to give 11. The highly-substituted
tetrahydropyran core 11 was then elaborated to the antifungal Botcinin F
(12).
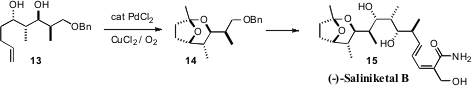
Ian Paterson of Cambridge University optimized
(Org. Lett. 2008, 10, 3295
DOI: 10.1021/ol801148d)
the Pd-catalyzed spirocyclization of the ene diol 13, leading to 14, the
enantiomerically-pure bicyclic core of (-)-Saliniketal B (15).
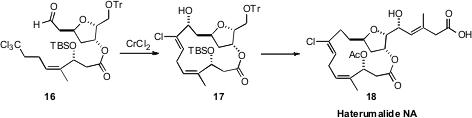
Haterumalide NA (18) presented the particular challenge of assembling the
geometrically-defined chloroalkene, in addition to closing the macrolide ring.
Babak Borhan of Michigan State University addressed
(J. Am. Chem. Soc. 2008, 130, 12228
DOI: 10.1021/ja8043695)
both of these challenges together, electing to employ a
chlorovinylidene chromium carbenoid, as developed by Falck and Mioskowski, to
effect the macrocyclization of 16 to 17.