Takahata), Elatol (Stoltz), 5-F2t-Isoprostane (Snapper), and
Ottelione B (Clive)
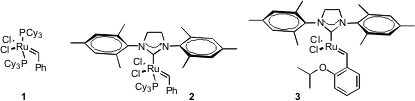
Alkene metathesis has been used to prepare more and more challenging natural
products. The first and second generation Grubbs catalysts 1 and 2 and the
Hoveyda catalyst 3 are the most widely used.
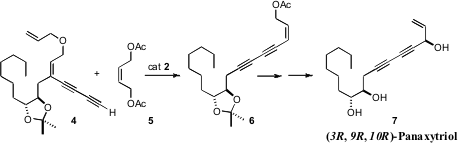
Daesung Lee of the University of Illinois at Chicago designed (Org. PMID:23357584 1867923-49-6 Chemscene Lett.
2008, 10, 257.
DOI: 10.1021/ol702651s)
a clever chain-walking cross metathesis, combining 4 and 5 to make
6. Price of 5-Bromopyridine-2-sulfonyl chloride The diyne 6 was carried on (3R, 9R, 10R)-Panaxytriol
(7).
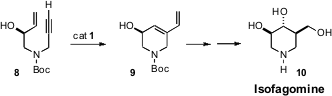
Tatsushi Imahori and Hiroki Takahata of Tohoku Pharmaceutical University
found (Tetrahedron Lett. 2008, 49, 265.
DOI: 10.1016/j.tetlet.2007.11.098)
that of the several derivatives
investigated, the unprotected alcohol 8 cyclized most efficiently. Selective
cleavage of the monosubstituted alkene followed by
hydroboration delivered the
alkaloid Isofagomine (10).
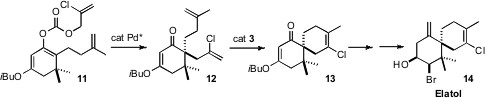
Brian M. Stoltz of Caltech established (J. Am. Chem. Soc. 2008,
130, 810.
DOI: 10.1021/ja710294k)
the absolute configuration of the halogenated chamigrene Elatol 14 using the
enantioselective enolate allylation that he had previously devised. A key
feature of this synthesis was the stereocontrolled preparation of the cis
bromohydrin.
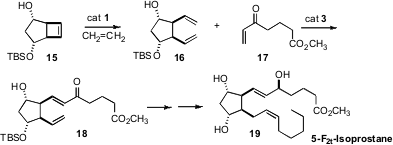
Marc L. Snapper of Boston College
opened (J. Org. Chem. 2008,
73, 3754
DOI: 10.1021/jo702702s)
the strained cyclobutene 15 with ethylene to give the diene 16. Remarkably, cross
metathesis with 17 delivered 18 with high regioselectivity, setting the stage
for the preparation of the 5-F2t-Isoprostane (19).
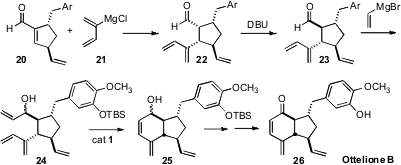
Derrick L. J. Clive of the University of Alberta assembled (J. Org. Chem.
2008, 73, 3078.
DOI: 10.1021/jo702635t)
Ottelione B (26) from the enantiomerically-pure aldehyde 20.
Conjugate addition of the
Grignard reagent 21 derived from chloroprene gave the
kinetic product 22, that was equilibrated to the more stable 23. Addition of vinyl Grignard followed by selective
ring-closing metathesis then led to
26.