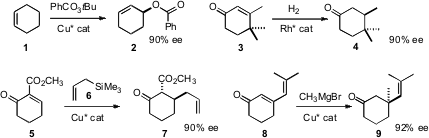
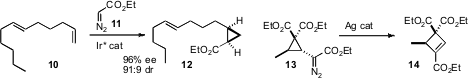Several elegant methods for the enantioselective transformation of preformed prochiral rings have been put forward. Derek R. Boyd of Queen’s University, Belfast devised(Chem. PMID:28739548 Commun. 2008, 5535.DOI: 10.1039/b814678k)a Cu catalyst that effected allylic oxidation of cyclic alkenes such as 1with high ee. Christoph Jäkel of the Ruprecht-Karls-Universität Heidelberg established(Adv. Synth. Catal. 2008, 350, 2708.DOI: 10.1002/adsc.200800462)conditions for the enantioselective hydrogenation of cyclic enones such as 3.Marc L. Snapper of Boston College developed(Angew. Formula of 4-Methylbenzene-1,3-diol 1-Bromo-4-(trifluoromethyl)benzene web Chem. Int. Ed. 2008, 47, 5049.DOI: 10.1002/anie.200800628)a Cu catalyst for the enantioselective allylation of activated cyclic enones such as 5. Alexandre Alexakis of the University of Geneva showed(Angew. Chem. Int. Ed. 2008, 47, 9122.DOI: 10.1002/anie.200803735)that dienones such as 8 could be induced to undergo1,4-addition, again with high ee.
Tsutomu Katsuki of Kyushu University originated(J. Am. Chem. Soc. 2008, 130, 10327.DOI: 10.1021/ja802561t)an Ir catalyst for the addition of diazoacetate 11 to alkenes such as 10 to give the cyclopropane 12 with high chemo-, enantio- and diastereoselectivity. Weiping Tang of the University of Wisconsin found(Angew. Chem. Int. Ed. 2008, 47, 8933.DOI: 10.1002/anie.200803910)a silver catalyst that rearranged cyclopropyl diazo esters such as 13 to the cyclobutene 14 with high regioselectivity.
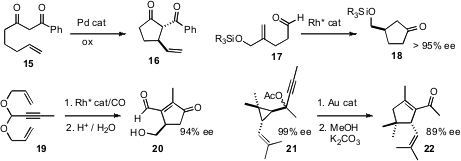
Zhang-Jie Shi of Peking University demonstrated(J. Am. Chem. Soc. 2008, 130, 12901.DOI: 10.1021/ja803452p)that under oxidizing conditions, a Pd catalyst could cyclize 15 to 16. Sergio Castillón of the UniversitatRovira i Virgili, Tarragona devised(Org. Lett. 2008, 10, 4735.DOI: 10.1021/ol801791g)a Rh catalyst for the enantioselective cyclization of 17 to 18. Virginie Ratovelomanana-Vidal of the ENSCP Paris and Nakcheol Jeong of Korea University established(Adv. Synth. Catal. 2008, 350, 2695.DOI: 10.1002/adsc.200800514)conditions for the enantioselective intramolecular Pauson-Khand cyclization of19 to give, after hydrolysis, the cyclopentenone 20. Quanrui Wang of Fudan University, Cristina Nevado of the Universität Zürich and Andreas Goeke of Shanghai Givaudan described(Angew. Chem. Int. Ed. 2008, 47, 10110.DOI: 10.1002/anie.200804202)the Au-mediated rearrangement of 21 to give, after hydrolysis, the cyclopentene 22 with minimal racemization. An alternative Au process converted 21 into the correspondingcyclohexenone with high ee.
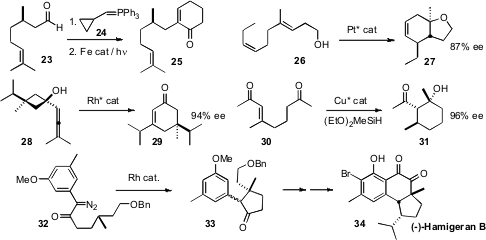
We developed(J. Org. Chem. 2008, 73, 8030.DOI: 10.1021/jo801767n)a general method for the conversion of an aliphatic aldehyde 23 to the cyclohexenone 25, condensation with the commercial phosphorane 24 followed by Fe-mediated cyclocarbonylation of the resulting alkenyl cyclopropane. Michel R. Gagné of the University of North Carolina devised(Angew. Chem. Int. Ed. 2008, 47, 6011.DOI: 10.1002/anie.200801423)a Pt catalyst for the cyclization of polyalkenes such as 26 in high ee.Nicolai Cramer of ETH Zurich found(Angew. Chem. Int. Ed. 2008, 47, 9294.DOI: 10.1002/anie.200804281)that ring expansion of the prochiral 28 to the cyclohexenone 29could be effected with substantial enantioselectivity. Bruce H. Lipschutz of the University of California, Santa Barbara extended(J. Am. Chem. Soc. 2008, 130, 14378.DOI: 10.1021/ja8045475)his enantioselective conjugate reduction to substrates such as 30, leading, via aldol condensation, to the cyclohexane 31.
Many methods for transition metal-mediatedpolycarbocyclic construction have also been reported. We showed(J. Org. Chem. 2008, 73, 7560.DOI: 10.1021/jo8010683)that cyclization of 32 gave 33, which we carried on to (-)-Hamigeran B (34).