As the computational methods used in pharmaceutical development have improved, receptor binding analysis has led to many potential new drug candidates that are polycyclic. N-Boc-PEG6-alcohol Data Sheet Such leads are often not pursued, however, because of the perception that even if it turned out to be active, an enantiomerically-pure polycyclic agent would be too expensive to manufacture. Taking this as a challenge, academic research groups continue to develop clever approaches for the efficient synthesis of complexpolycarbocyclic target structures. Three recent approaches are outlined here. Buy1314649-82-5
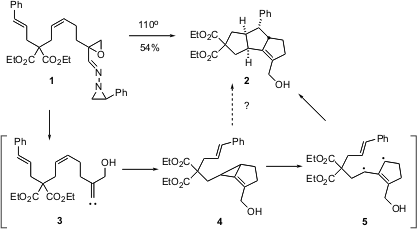
Hee-Yoon Lee of the Korea Advanced Institute of Science & Technology (KAIST) in Daejon reported (J. Am. PMID:24140575 Chem. Soc. 2003, 125, 10156.DOI: 10.1021/ja036263l)that on heating, the imine 1 is cleanly converted into the tricyclic 2. The reaction presumably proceeds via insertion of the alkylidene carbene 3 into the alkene, to give the unstable alkylidene cyclopropane 4. The authors suggest that 4 opens to the diradical 5, which then cyclizes. It is striking that the geometry of the starting alkene 1 is retained in the product 2. It is possible that in fact there is a concerted pathway for the opening of 4 and simultaneous insertion, to give 2.
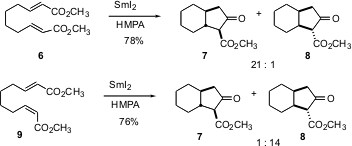
Hiroto Nagaoka of Meiji Pharmaceutical University has reported (Tetrahedron Lett. 2003, 44, 4649.DOI: 10.1016/S0040-4039(03)01093-1)the tandem reduction – Dieckmann cyclization of the esters 6 and 9. It is striking that the geometry of the starting alkene dictates the ring fusion of the product. Both 5/5 and 6/5 systems can be prepared this way.
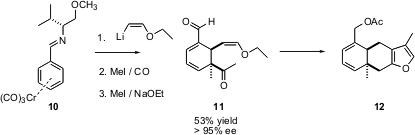
The furanoterpene 15-acetoxytubipofuran 12 shows cytotoxicity against B-16 melanoma cells. E. Peter Kündig of the University of Geneva has reported (J. Am. Chem. Soc. 2003, 125, 5642.DOI: 10.1021/ja029957n)a concise asymmetric synthesis of12, based on the addition of lithio ethyl vinyl ether to the chromium tricarbonyl-activated benzaldehyde imine10. In the course of the organometallic addition, five carbon-carbon bonds are formed.