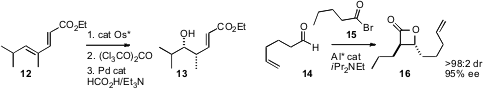The Jørgensen Synthesis of the Autoregulator IM-2
Amando Córdova of Stockholm University found (Angew. Chem. Int. Ed.
2008, 47, 8468.
DOI: 10.1002/anie.200802335)
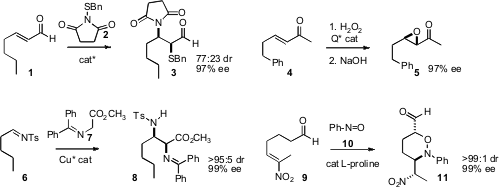
that the enantiomerically-enriched diastereomers from
aminosulfenylation of 1 were readily separable by silica gel
chromatography. Benjamin List of the Max-Planck-Institut, Mülheim developed
(Angew. Chem. Int. Ed. 2008, 47, 8112.
DOI: 10.1002/anie.200803238)
what appears to be a general protocol for the enantioselective
epoxidation
of enones such as 4. Paolo Melchiorre of the Università di Bologna devised
(Angew. Chem. Int. Ed. 2008, 47, 8703.
DOI: 10.1002/anie.200803647)
a related protocol for the enantioselective
aziridination of enones. Xue-Long Hue of the Shanghai Institute of Organic Chemistry and Yun-Dong Wu
of the Hong Kong University of Science and Technology optimized
(J. Am. Chem. Soc. 2008, 130, 14362.
DOI: 10.1021/ja804527r)
a Cu catalyst for enantioselective
Mannich homologation of imines such as
6. Guofu Zhong of Nanyang Technological University, Singapore established
(Angew. Chem. Int. Ed. PMID:28038441 2008, 47, 10187,
DOI: 10.1002/anie.200803731;
Org. Lett. 2008, 10, 4585,
DOI: 10.1021/ol801864c)
that enantioselective α-aminoxylation of an ω-alkenyl aldehyde
such as 9 could lead to defined arrays of stereogenic centers. 6-Bromo-7-methoxyquinazolin-4(1H)-one Chemical name 942920-50-5 In stock
George A. O’Doherty of West Virginia University devised
(Org. Lett. 2008, 10, 3149.
DOI: 10.1021/ol801055b)
a protocol for the enantioselective hydration of 12 to 13. René
Peters of the University of Stuttgart designed
(Angew. Chem. Int. Ed. 2008, 47, 5461.
DOI: 10.1002/anie.200801143)
an Al catalyst for the enantioselective combination of an acyl bromide 15 with an
aldehyde14 to deliver the β-lactone 16. Hajime Ito and Masaya Sawamura
of Hokkaido University established
(J. Am. Chem. Soc. 2008, 130, 15774.
DOI: 10.1021/ja806602h)
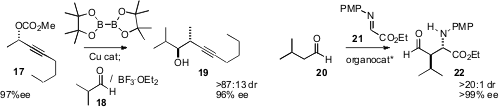
that the allenyl borane from 17 added to aldehydes such as 18 with high ee.
Keiji Maruoka of Kyoto University developed
(Tetrahedron Lett. 2008, 49, 5369.
DOI: 10.1016/j.tetlet.2008.06.093)
an organocatalyst for the Mannich
homologation of an aldehyde such as 20 to 21.
R. Karl Dieter of Clemson University showed
(Org. Lett. 2008, 10, 2087.
DOI: 10.1021/ol800727f)
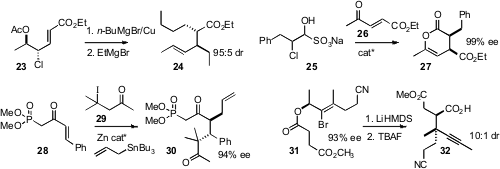
that 23,readily prepared in high ee, could be displaced sequentially with two
different Grignard reagents, to give 24. Jeffrey W. Bode, now at the University
of Pennsylvania, found
(Org. Lett. 2008, 10, 3817.
DOI: 10.1021/ol801502h)
that bisulfite adducts such as 25 served well for the addition of unstable
chloroaldehydes to 26 to give 27. Sunggak Kim of KAIST, Daejeon observed
(Org. Lett. 2008, 10, 3149.
DOI: 10.1021/ol801055b)
that the radical addition of 29 to 28 with allylation of the intermediate radical
delivered 30 with high diastereocontrol. Peter A. Jacobi of Dartmouth College prepared
(Org. Lett. 2008, 10, 2837.
DOI: 10.1021/ol800985m)
the ester 31 by enantioselective reduction of the enone.
Claisen rearrangement
followed by TBAF elimination gave 32.
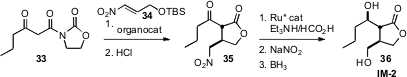
Karl Anker Jørgensen of Aarhus University, Denmark used
(Chem. Commun. 2008, 5827.
DOI: 10.1039/b812698d)
a Chincona based catalyst to effect enantioselective addition of 33 to
34. Enantioselective reduction of the ketone 35 to the alcohol followed by
conversion of the nitro group to the alcohol led to IM-2 (36), an autoregulator
derived from Streptomyces.
It is a measure of the progress that has been made in this area that fifteen
years ago, developing a route to a family of drug products that contained two
adjacent ternary alkylated stereogenic centers was a significant challenge for
the process group of a major pharmaceutical company.