(MacMillan)
Yoshiji Takemoto of Kyoto University designed (Org. 288617-73-2 Chemscene Lett. 2009, 11, 2425.
DOI: 10.1021/ol9006053)
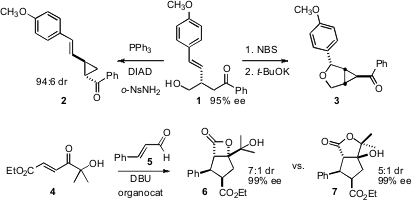
an organocatalyst for the enantioselective conjugate addition
of alkene boronic acids to γ-hydroxy enones, leading to 1 in high ee. Attempted
Mitsunobu coupling led to the cyclopropane 2, while bromoetherification followed
by intramolecular alkylation delivered the cyclopropane 3.
Jeffrey W. Bode of the University of Pennsylvania demonstrated
(Org. 943719-62-8 Formula Lett. 2009, 11, 677.
DOI: 10.1021/ol802739d)
a remarkable dichotomy in the reactivity of N-heterocyclic
carbenes. A triazolium precatalyst combined 4 and 5 to give 6, whereas an
imidazolium precatalyst combined 4 and 5 to give 7.
Xinmiao Liang of the Dalian Institute of Chemical Physics and Jinxing Ye of
the East China University of Science and Technology devised
(Org. PMID:32472497 Lett. 2009, 11, 753.
DOI: 10.1021/ol802892h)
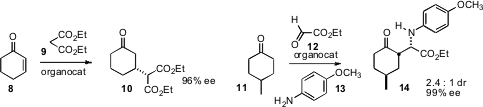
a Cinchona-derived catalyst that converted the prochiral
cyclohexenone
8 into the diester 10 in high ee. Rich G. Carter of Oregon State University found
(J. Org. Chem. 2009, 74, 2246.
DOI: 10.1021/jo8027938)
a simple sulfonamide-based proline catalyst that effected the
Mannich condensation of the prochiral
ketone with ethyl glyoxalate 12 and the amine 13, leading to the amine 14.
In the first pot of a concise, three-pot synthesis of (-)-oseltamivir, Yujiro
Hayashi of the Tokyo University of Science combined
(Angew. Chem. Int. Ed. 2009, 48, 1304.
DOI: 10.1002/anie.200804883)
15 and 16 in the presence of a catalytic amount of diphenyl prolinol
TMS ether to give an intermediate nitro aldehyde. Addition of the phosphonate 17 led to
a cyclohexenecarboxylate, that on the addition of the thiophenol 18
equilibrated to the ester 19. Ying-Chun Chen of Sichuan University used
(Org. Lett. 2009, 11, 2848.
DOI: 10.1021/ol9010568)
a related diaryl prolinol TMS ether to direct the condensation of the readily-prepared
phosphorane 20 with the unsaturated aldehyde 21 to give the cyclohexenone
22. Armando Córdova of Stockholm University also used
(Tetrahedron Lett. 2009, 50, 3458.
DOI: 10.1016/j.tetlet.2009.02.209)
diphenyl prolinol TMS ether to mediate the addition of 24 to 23.
The subsequent intramolecular aldol condensation proceeded with high diastereocontrol,
leading to 25.
Benjamin List of the Max-Planck Institut, Mülheim employed
(Nat. Chem. 2009, 1, 225.
DOI: 10.1038/nchem.215)
a MacMillan catalyst for the reductive cyclization of 26. Subsequent
epimerization and Tishchenko hydride transfer then proceeded with high
diastereoselectivity, leading directly to the liverwort-derived (+)-Ricciocarpin
A (28).
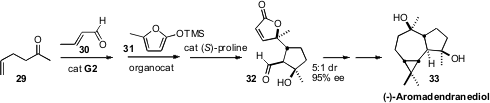
David W. C. MacMillan of Princeton University devised
(Angew. Chem. Int. Ed. 2009, 48, 4349.
DOI: 10.1002/anie.200900220)
a remarkable one-pot three-component assembly of the lactone
32.
Cross-metathesis of 29 with 30 gave a keto aldehyde. Direct addition of the
furan 31 and an chiral imidazolidinone catalyst effected
conjugate addition to
the unsaturated aldehyde. A third catalyst, (S)-proline, then mediated
intramolecular aldol condensation. The crystalline lactone 32 was readily
carried on to (-)-Aromadendranediol (33).