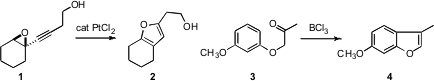Masahiro Yoshida of the University of Tokushima described(Tetrahedron Lett. 2008, 49, 5021.DOI: 10.1016/j.tetlet.2008.06.043)the Pt-mediated rearrangement of alkynyl oxiranes such as 1to the furan 2. Roman Dembinski of Oakland University reported(J. Org. Chem. PMID:24635174 2008, 73, 5881.DOI: 10.1021/jo8007995)a related zinc-mediated rearrangement of propargyl ketones to furans. Buy6-Chlorobenzo[a]phenazin-5-ol The cyclization of aryloxy ketones such as 3 to the benzofuran 4 developed(Tetrahedron Lett. 2212021-40-2 Data Sheet 2008, 49, 6579.DOI: 10.1016/j.tetlet.2008.09.034)by Ikyon Kim of the Korea Research Institute of Chemical Technology islikely proceeding by a Friedel-Crafts mechanism.
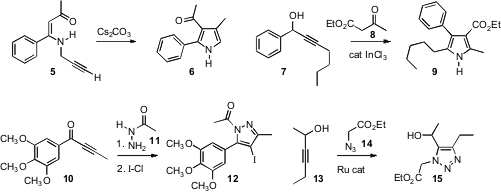
Sandro Cacchi and Giancarlo Fabrizi of Università degli Studi “La Sapienza”, Roma, observed(Org. Lett. 2008, 10, 2629.DOI: 10.1021/ol800518j)that base converted the enamine 5 to the pyrrole 6. Alternatively, oxidation of 5 with CuBr led to a pyridine. Zhuang-ping Zhuan of Xiamen University prepared(Adv. Synth. Catal. 2008, 350, 2778.DOI: 10.1002/adsc.200800473)pyrroles such as 9 by condensing an alkynyl carbinol 7 with a1,3-dicarbonyl compound. Richard C. Larock of Iowa State University found(J. Org. Chem. 2008, 73, 6666.DOI: 10.1021/jo800789p) that combination of an alkynyl ketone 10 with 11 followed by oxidation with I-Cl led to the pyrazole 12.
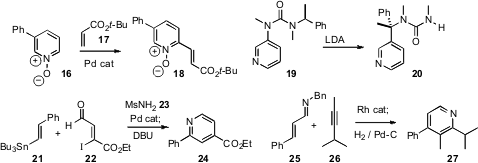
The “click” condensation of azides with alkynes, leading to the 1,4-disubstituted 1,2,3-triazole, has proven to be a powerful tool for combinatorial synthesis. Valery V. Fokin of Scripps/La Jolla and Zhenyang Lin and Guochen Jia of the Hong Kong University of Science and Technology have developed(J. Am. Chem. Soc. 2008, 130, 8923.DOI: 10.1021/ja0749993)a complementary approach, using Ru catalysts to prepare 1,5-disubstituted 1,2,3-triazoles. Remarkably, internal alkynes participate, and, as in the conversion of 13 to 15, propargylic alcohols direct the regioselectivity of the cycloaddition.
A variety of methods have been put forward for functionalizing pyridines. Sukbok Chang of KAIST described(J. Am. Chem. Soc. 2008, 130, 9254.DOI: 10.1021/ja8026295)the direct oxidative homologation of a pyridine N-oxide 16 to give the unsaturated ester 18. Jonathan Clayden of the University of Manchester observed(Org. Lett. 2008, 10, 3567.DOI: 10.1021/ol801332n)that metalation of 19 gave an anion that rearranged to 20 with complete retention of enantiomeric excess.
Shigeo Katsumura of Kwansei Gakuin University developed(Tetrahedron Lett. 2008, 49, 4349.DOI: 10.1016/j.tetlet.2008.05.048)an intriguing three-component coupling, combining 21, 22,and methanesulonamide 23 to give the pyridine 24. Robert G. Bergman and Jonathan A. Ellman of the University of California, Berkeley established(J. Am. Chem. Soc. 2008, 130, 3645.DOI: 10.1021/ja7104784)a C-H activation based coupling, combining 25 and 26 to give, after debenzylation/aromatization, the pyridine 27.
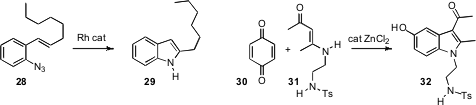
Tom G. Driver of the University of Illinois, Chicago found(Angew. Chem. Int. Ed. 2008, 47, 5056.DOI: 10.1002/anie.200800689)that Rh octanoate catalyzed the well-known thermal conversion of an azide such as 28 to the indole 29. Valeriya S. Velezheva of the A. N. Nesmeyanov Institute of Organoelement Compounds uncovered(Tetrahedron Lett. 2008, 49, 7106.DOI: 10.1016/j.tetlet.2008.09.087)an improved protocol for the Nenitzescu synthesis, combining 30 and 31 to give 32. For an overview of the nine types of indole synthesis, see our upcoming review in Angewandte Chemie.