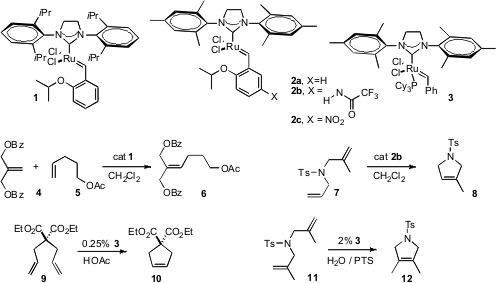
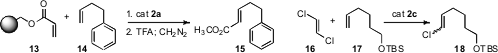As alkene metathesis is extended to more and more challenging substrates, improved catalysts and solvents are required. Robert H. 233276-38-5 manufacturer Grubbs of Caltech developed (Org. Lett. tBuBrettPhos Pd G3 Chemical name 2008, 10, 441.DOI: 10.1021/ol702624n)the diisopropyl complex 1, that efficiently formed the trisubstituted alkene 6 bycross metathesis of 4 with 5. Hervé Clavier and Stephen P. Nolan of ICIQ, Tarragona, and Marc Mauduit of ENSC Rennes found (J. Org. Chem. 2008, 73, 4225.DOI: 10.1021/jo800203d)that after cyclization of 7 with the complex 2b, simple filtration of the reaction mixture through silica gel delivered the product 8 containing only 5.5 ppm Ru. PMID:23554582
The merit of CH2Cl2 as a solvent for alkene metathesis is that the catalysts (e.g. 1-3) are very stable. Claire S. Adjiman of Imperial College and Paul C. Taylor of the University of Warwick established (Chem. Commun. 2008, 2806.DOI: 10.1039/b802921k)that although the second generation Grubbs catalyst 3 is not as stable in acetic acid as a reaction solvent, for the cyclization of 9 to 10 it is a much more active catalyst in acetic acid than in CH2Cl2. Bruce H. Lipshutz of the University of California, Santa Barbara observed(Adv. Synth. Catal. 2008, 350, 953.DOI: 10.1002/adsc.200800114)that even water could serve as the reaction solvent for the challenging cyclization of 11 to 12, so long as the solubility-enhancing amphiphile PTS was included.
Ernesto G. Mata of the Universidad Nacional de Rosario explored (J. Org. Chem. 2008, 73, 2024.DOI: 10.1021/jo7025433)resin isolation to optimize cross-metathesis, finding that the acrylate 13 worked particularly well. Karol Grela of the Polish Academy of Sciences, Warsaw optimized (Chem. Commun. 2008, 2468.) cross-metathesis with a halogenated alkene 16.
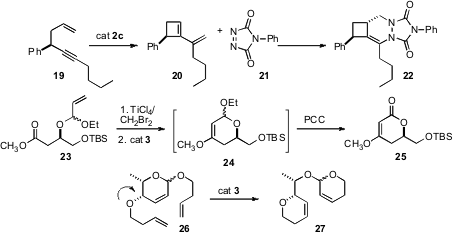
Jean-Marc Campagne of ENSC Montpellier extended (J. Am. Chem. Soc. 2008, 130, 1562DOI: 10.1021/ja0780986)ring-closing metathesis to enynes such as 19. The product diene 20 was a reactive Diels-Alder dienophile. István E. Markó of the Université Catholique de Louvain applied (Tetrahedron Lett. 2008, 49, 1523.DOI: 10.1016/j.tetlet.2007.12.113)the known (![]() 2007, January 22) ring-closing metathesis of enol ethers to the cyclization of the Tebbe product from 23. The ether24 was oxidized directly to the lactone 25. Jacques Eustache of the Université de Haute-Alsace observed (Tetrahedron Lett. 2008, 49, 1192.DOI: 10.1016/j.tetlet.2007.12.047)that ring-opening/ring-closing metathesis proceeded efficiently with 26, but not with the ether epimeric at the indicated position.
2007, January 22) ring-closing metathesis of enol ethers to the cyclization of the Tebbe product from 23. The ether24 was oxidized directly to the lactone 25. Jacques Eustache of the Université de Haute-Alsace observed (Tetrahedron Lett. 2008, 49, 1192.DOI: 10.1016/j.tetlet.2007.12.047)that ring-opening/ring-closing metathesis proceeded efficiently with 26, but not with the ether epimeric at the indicated position.
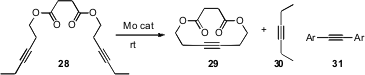
Jeffrey S. Moore of the University of Illinois prepared (J. Org. Chem. 2008, 73, 4256.DOI: 10.1021/jo8003919) a Mo alkyne metathesis catalyst on fumed silica gel that converted 28 to 29 at room temperature. The cyclization was driven by the vacuum removal of 3-hexyne 30. In the same paper, Professor Moore used the insolubility of a product diaryl alkyne 31 to drive other cyclizations.