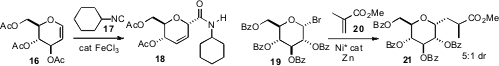(+)-Omaezakianol
Tobin J. Marks of Northwestern University observed
(J. Am. Chem. Soc. 2009, 131, 263.
DOI: 10.1021/ja8072462)
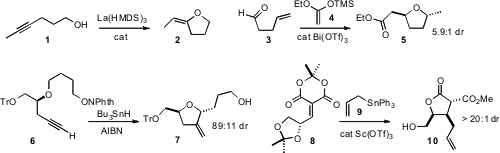
high geometric control in the cyclization of 1
to 2. Tristan H. Lambert of Columbia University found
(Org. Lett. 2009, 11, 1381.
DOI: 10.1021/ol900198r)
that Bi could catalyze both the addition of the
ketene silyl acetal 4 to 3, and the subsequent cyclization of the
secondary alcohol so formed, to give the product ether 5 with high
diastereocontrol. Glenn M. Sammis of the University of British Columbia devised
(Org. Lett. 2009, 11, 2019.
DOI: 10.1021/ol900481e)
a radical relay cyclization of 6 to 7, again with high diastereocontrol.
Eric Fillion of the University of Waterloo established
(Org. Lett. 2009, 11, 1919.
DOI: 10.1021/ol9003959)
that conjugate addition to the Meldrum’s acid derivative 8 proceeded with
high stereoselectivity, delivering the useful chiron 10.
Gregory C. 152835-00-2 Formula Fu of MIT found
(Angew. PMID:25959043 Price of 144740-56-7 Chem. Int. Ed. 2009, 48, 2225.
DOI: 10.1002/anie.200805377)
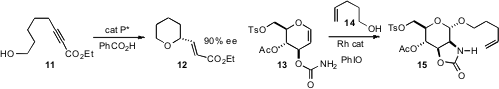
that both five- and six-membered ring ethers could be formed with high
enantiocontrol from alkyne alcohols such as 11. The catalyst was a chiral
phosphine. Christian M. Rojas of Barnard College established
(Org. Lett. 2009, 11, 1527.
DOI: 10.1021/ol900126q)
a route to 2-amino sugars such as 15, by Rh-mediated intramolecular
nitrene addition in the presence of the trapping agent 14. J. S.
Yadav of the Indian Institute of Chemical Technology, Hyderabad devised
(Tetrahedron Lett. 2009, 50, 81.
DOI: 10.1016/j.tetlet.2008.10.090)
a route to C-glycosides such as 18, by condensation of a glycal 16 with
an isonitrile 17. Michel R. Gagné of the University of North Carolina developed
(Org. Lett. 2009, 11, 879.
DOI: 10.1021/ol8028737)
a complementary route to C-glycosides such as 21, with control of side chain relative
configuration. Note that the addition to the methacrylate 20 is likely
proceeding by initial one-electron reduction, since reductive β-elimination is
not observed.
It is also possible to construct larger rings. Frank E. McDonald of Emory
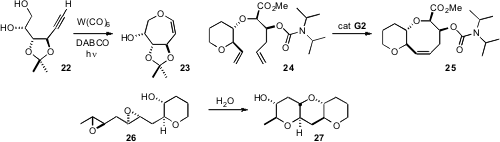
University devised a flexible route to protected tetraols such as 22, and showed
(Org. Lett. 2009, 11, 851.
DOI: 10.1021/ol8028065)
that it could be cyclized selectively to the septanoside 23.
Kenshu Fujiwara of Hokkaido University found
(Tetrahedron Lett. 2009, 50, 1236.
DOI: 10.1016/j.tetlet.2009.01.011)
that ring-closing metathesis of 24 delivered the eight-membered
ring product 25 in near quantitative yield.
For the synthesis of the ladder ethers, six-membered ring formation, as illustrated
by the cyclization of 26 to 27, is required. Timothy F. Jamison of MIT found
(J. Am. Chem. Soc. 2009, 131, 6678,
DOI: 10.1021/ja9025243;
Angew. Chem. Int. Ed. 2009, 48, 4430,
DOI: 10.1002/anie.200900924)
that six-membered ring formation can best be accomplished if the
cyclization is carried out in water, without catalyst. The preference for
six-membered ring formation is still dominant even in cases where methyl
substitution would usually direct five-membered ring formation.
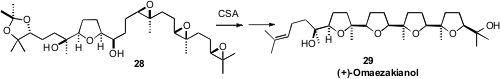
Acid-catalyzed cyclization of polyepoxides such as 28 strongly favors
five-membered ring formation. In that cyclization, the central reaction in the
synthesis of (+)-omaezakianol (29) reported
(Angew. Chem. Int. Ed. 2009, 48, 2538.
DOI: 10.1002/anie.200805857)
by Yoshiki Morimoto of Osaka City University, three of the four tetrahydrofuran
rings of 29 are formed in a single step, each with high stereocontrol.