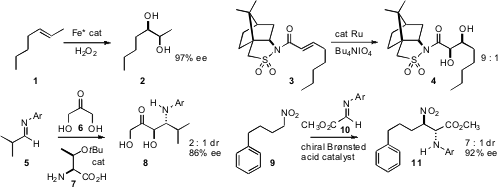
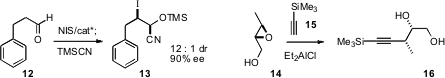The Sharpless osmium-catalyzed asymmetric dihydroxylation is widely used. Lawrence Que, Jr. of the University of Minnesota designed(Angew. Chem. Int. Ed. 2008, 47, 1887DOI: 10.1002/anie.200705061)a catalyst with the inexpensive Fe that appears to be at least as effective, converting 1 to 2 in high ee. In an alternative approach,Bernd Plietker of the Universität Stuttgart used(J. Org. Chem. 2008, 73, 3218DOI: 10.1021/jo800145x)chiral auxiliary control to direct dihydroxylation. The diastereomers of 4 were readily differentiated. 2611225-93-3 Chemscene
Defined arrays of stereogenic centers can also be constructed by homologation. Armando Córdova of Stockholm University condensed (Tetrahedron Lett. 2008, 49, 803DOI: 10.1016/j.tetlet.2007.11.196)dihydroxy acetone 6 with an in situ generated imine 5 to give the amino diol 8. In parallel work, Carlos F. Barbas III of Scripps/La Jolla described(Org. Buy53103-03-0 Lett. 2008, 10, 1621DOI: 10.1021/ol8002833)a related addition to aldehydes. Magnus Rueping of University Frankfurt found(Org. Lett. PMID:24428212 2008, 10, 1731DOI: 10.1021/ol8003589)conditions for the addition of a nitro alkane such as 9 to the imine10 to give 11.
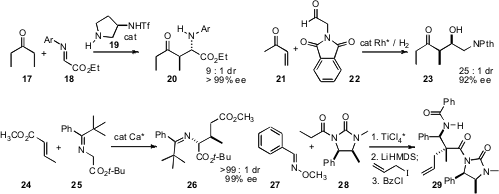
Keiji Maruoka of Kyoto University devised(J. Am. Chem. Soc. 2008, 130, 3728DOI: 10.1021/ja074003o)a chiral amine that mediated the enantioselectiveiodination of aldehydes such as 12. Direct cyanohydrin formation delivered 13 in high de and ee. The epoxide 14 is readily prepared in high ee from crotyl alcohol. Barry M. Trost of Stanford University found(Org. Lett. 2008, 10, 1893DOI: 10.1021/ol800347u)that 14 could be opened with 15, to give 16 with high regio- and diastereocontrol.
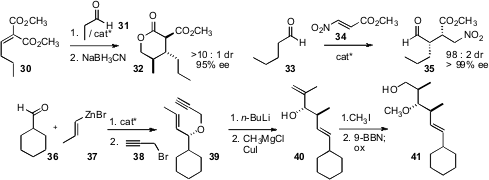
Jérôme Blanchet of the Université de Caen Basse-Normandie optimized(Org. Lett. 2008, 10, 1029DOI: 10.1021/ol8000975)the amine 19 as a catalyst for the condensation of ketones such as17 with the imine 18, to give20. Michael J. Krische of the University of Texas has explored(J. Am. Chem. Soc. 2008, 130, 2746DOI: 10.1021/ja710862u)the in situ generation of chiral Rh enolates from enones such as 21,and the subsequent aldol condensation with aldehydes such as 22.
Shu Kobayashi of the University of Tokyo found(Org. Lett. 2008, 10, 807DOI: 10.1021/ol702958w)that the conjugate addition of 25 to 24 mediated by a chiral Ca catalyst proceeded with high enantiocontrol at both of the newly formed stereogenic centers, to give 26. In a chiral auxiliary based approach, Dennis C. Liotta found(J. Org. Chem. 2008, 73, 1264DOI: 10.1021/jo7018202)that condensation of 27 with 28 gave predominantly just two of the possible four diastereomeric azetines. Alkylation of the cis diastereomer, followed by benzoylation and hydrolysis, then delivered the α-quaternary β-amino acid derivative 29 as a single enantiomerically-pure diastereomer.
Professor Córdova showed(Adv. Synth. Catal. 2008, 350, 657DOI: 10.1002/adsc.200700570)that aldehydes could be added to alkylidene malonates such as 30 to give, after reduction, the lactone 32.Dawei Ma of the Shanghai Institute of Organic Chemistry found(Angew. Chem. Int. Ed. 2008, 47, 545DOI: 10.1002/anie.200704161)that aldehydes could also be added to nitroalkenes such as 34 withhigh enantio- and diasterocontrol. Kathlyn A. Parker of SUNY Stony Brook took advantage(Org. Lett. 2008, 10, 1349DOI: 10.1021/ol702989g)of the enantioselective addition of an alkenyl zinc halide 37 to an aldehyde 36 toset the relative and absolute configuration of an extended array of stereogenic centers.