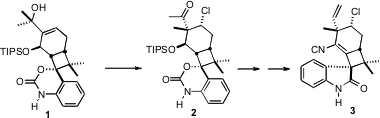
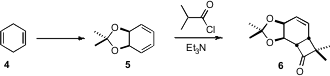Welwitindolinone A Isonitrile (3) is the first of a family of
oxindole natural products isolated from the cyanobacteria Hapalosiphon welwischii
and Westiella intricate on the basis of their activity for reversing
multiple drug resistance (MDR). A key transformation in the total synthesis of
3 reported (J. Am. Chem. Soc. PMID:24580853 Formula of 349552-70-1 2008, 130, 2087
DOI: 10.1021/ja057640s)
by John L. 4,4′-Dibromo-2,2′-bipyridine supplier Wood, now at Colorado State University, was the chlorination of 1,
that in one step established both the axial secondary chloro substituent and the
flanking chiral quaternary center.
The starting material for the synthesis of 3 was the diene
acetonide
5, readily prepared from the
Birch reduction product 4.
Intermolecular ketene cycloaddition proceeded with high regio- and
diastereoselectivity, to give the bicyclooctenone 6.
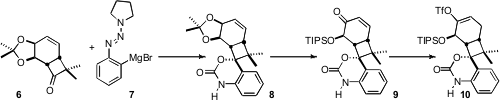
The triazene-bearing Grignard reagent 7 added to the ketone 6
with the anticipated high diastereocontrol, to give, after reduction and
protection, the cyclic urethane 8. Selective oxidation of the diol
derived from 8 followed by silylation delivered the enone 9.
Conjugate addition of hydride followed by enolate trapping gave the triflate
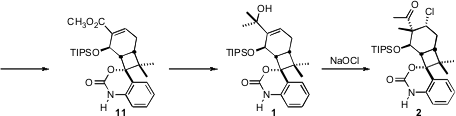
10. Pd-catalyzed methoxycarbonylation established the methyl ester 11.
Addition of CH3MgBr to 11 gave 1, setting the stage for
the establishment of the two key stereogenic centers of 2 and so of 3.
The transformation of 1 to 2 was envisioned as being initiated
by formation of a bridging chloronium ion.
Pinacol-like 1,2-methyl migration
then proceeded to form the trans diaxial product, moving the ketone-bearing
branch equatorial. In addition to being an elegant solution of the problem of
how to establish the axial chloro substituent of 3, this strategy might
have some generality for the stereocontrolled construction of other alkylated
cyclic quaternary centers.
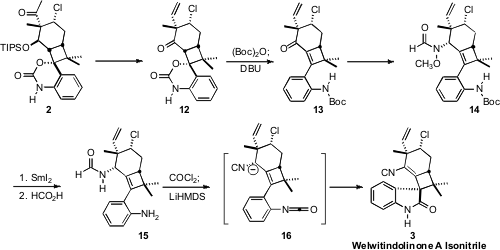
Reduction of the ketone 2 and dehydration of the resulting alcohol led,
after deprotection and oxidation, to the ketone 12. Protection followed
by β-elimination gave the enone 13. Direct reductive amination of 13 failed, but
reduction of the methoxime was successful, giving, after acylation, the
formamide 14. Reductive N-O bond cleavage followed by deprotection and
isonitrile formation then set the stage for the planned intramolecular acylation
to complete the synthesis of Welwitindolinone A Isonitrile (3).
The starting diene 5 used in this synthesis was prochiral, leading to racemic
3. Now that the route to 3 is established, it would be interesting to devise a
method for preparing enantiomerically-enriched 6. Enantiomerically-pure variants
of 5 have been prepared, inter alia by fermentation of halogenated aromatics.
Alternatively, an enantioselective version of the [2+2] cycloaddition to the
prochiral 5 could be developed.