Rich G. PMID:23381626 Carter of Oregon State University described
(J. Am. Chem. Soc. 2008, 130, 9238
DOI: 10.1021/ja803613w)
the first enantioselective synthesis of the
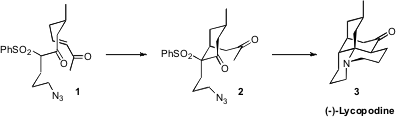
Lycopodium alkaloid (-)-lyopodine (3). A key step in the assembly of 3
was the diastereoselective intramolecular
Michael addition of the keto sulfone
of 1 to the enone, leading to the
cyclohexanone 2. 1246761-84-1 structure
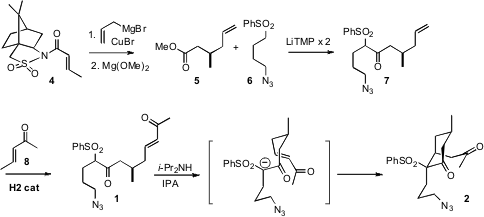
The key cyclization substrate 1 bore a single secondary methyl group. 942518-20-9 Formula
While that could have been derived from a natural product, it was operationally
easier to effect chiral auxiliary controlled conjugate addition to the crotonyl
amide 4, leading, after methoxide exchange, to the ester 5. The
authors reported that double deprotonation with LiTMP gave superior results, vs.
LDA or BuLi, in the condensation of 6 with 5 to give 7.
Metathesis with pentenone 8 gave the intramolecular Michael substrate
1.
The authors thought that they would need a chiral catalyst to drive the
desired stereocontrol in the cyclization of 1 to 2. As a control,
they tried an achiral base first, and were pleased to observe the desired
diastereomer crystallize from the reaction mixture in 89% yield. The structure
of 2 was confirmed by X-ray crystallography.
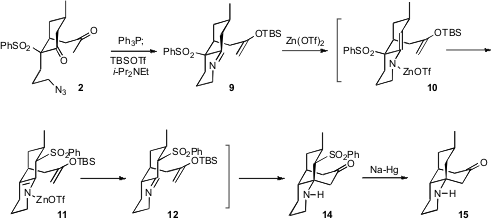
To prepare for the intramolecular
Mannich condensation, the azide was reduced
to give the imine, and the methyl ketone was converted to the silyl enol ether.
Under Lewis acid conditions, the sulfonyl group underwent an unanticipated
1,3-migration, to give 11. Cyclization of 12 then delivered the
crystalline 14. Reduction converted 14 to the known (in racemic
form) ketone 15.
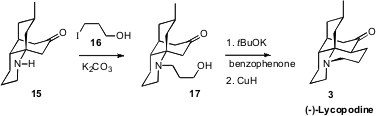
To complete the synthesis, the amine 15 was alkylated with 16
to give the alcohol 17.
Oppenauer oxidation followed by aldol
condensation delivered the cyclized enone, that was reduced with the Stryker
reagent to give (-)-Lycopodine (3).
Both the cyclization of 1 to 2 and the cyclization of 9
to 14 are striking. It may be that the steric demand of the
phenylsulfonyl group destabilizes the competing transition state for the
cyclization of 1.