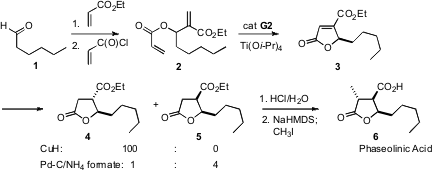
As N. Azido-PEG8-acid Purity Selvakumar of Dr. Reddy’s Laboratories, Ltd., Hyderabad approached (Tetrahedron Lett. 2007, 48, 2021.DOI: 10.1016/j.tetlet.2007.01.053)the synthesis of phaseolinic acid (6), there was some concern about the projected cyclization of 2 to 3, as this would involve the coupling of two electron-deficient alkenes. PMID:23865629 In fact, the Ru-mediatedring-closing metathesis proceeded efficiently. Fmoc-L-Val-OH Data Sheet The product unsaturated lactone3 could be reduced selectively to either the trans product 4 or the cis product 5.
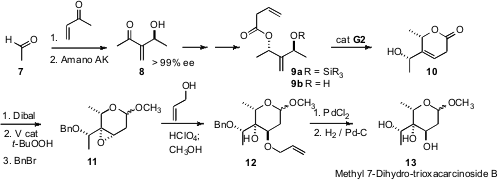
There has been relatively little work on the synthesis of the higher branched sugars, such as the octalose (13), a component of several natural products. The synthesis of 13 (Org. Lett. 2007, 9, 4777. DOI: 10.1021/ol702078t)by Ulrich Koert of the Philipps-University Marburg also began with a Baylis-Hillman product, the easily-resolved secondary alcohol 8. As had been observed in other contexts, cyclization of the protected allylic alcohol 9a failed, but cyclization of the free alcohol 9b proceeded smoothly. V-directedepoxidation then set the relative configuration of the stereogenic centers on the ring.
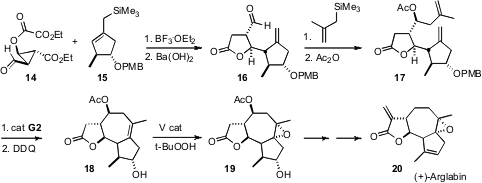
Ring-closing metathesis to construct tetrasubstituted alkenes has been a challenge, and specially-designed Ru complexes have been put forward specifically for this transformation. Oliver Reiser of the Universität Regensburg was pleased to observe (Angew. Chem. Int. Ed. 2007, 46, 6361. DOI: 10.1002/anie.200701584)that the second-generation Grubbs catalyst itself worked well for the cyclization of 17 to 18. Again in this synthesis, catalytic V was used to direct the relative configuration of the epoxide.
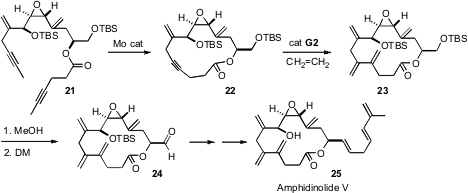
Intramolecular alkyne metathesis is now well-established as a robust and useful method for organic synthesis. It was also known that Ru-mediated metathesis of an alkyne with ethylene could lead to the diene. The question facing (Angew. Chem. Int. Ed. 2007, 46, 5545.DOI: 10.1002/anie.200701640)Alois Fürstner of the Max-Planck-Institut, Mülheim was whether these transformations could be carried out on the very delicate epoxy alkene 21. In fact, the transformations of 21 to 22 and of 22 to 23 proceeded well, setting the stage for the total synthesis of Amphidinolide V (25).