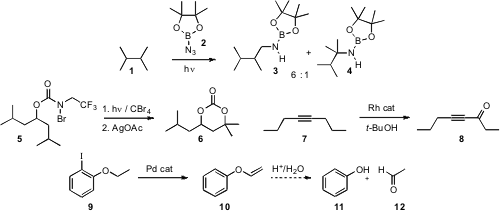
A classic example of C-H functionalization is the familiarNBSbromination of a benzylic site. Recent updates of this approach allow for direct alkoxylation(J. Am. Chem. Soc. 2008, 130, 7824DOI: 10.1021/ja8031218)and net amination (Org. Lett. 2008, 10, 1863.DOI: 10.1021/ol800593p)For the amination of simple aliphatic H’s, Holger F. Bettinger of Ruhr-Universität Bochum developed(Angew. Chem. Int. Ed. PMID:35567400 2008, 47, 4744.DOI: 10.1002/anie.200705936)the boryl azide 2. The insertion with 1 proceeded to give a statistical mixture of the nitrene insertion products 3 and 4. The tethered C-H functionalization devised (J. Am. Chem. 204058-25-3 manufacturer Soc. 2008, 130, 7247.DOI: 10.1021/ja802491q)by Phil S. 3-Amino-5-(tert-butyl)phenol site Baran of Scripps-La Jolla is selective, as in the conversion to 5 to 6, but appears to be limited to tertiary and benzylic C-H sites.
Michael P. Doyle of the University of Maryland established (J. Org. Chem. 2008, 73, 4317.DOI: 10.1021/jo800382p)an elegant protocol for the oxidation of an alkyne such as 7 to the ynone 8. Note that the oxidation did not move the alkyne. Marta Catellani of the Università di Parma reported (Adv. Synth. Catal. 2008, 350, 565.DOI: 10.1002/adsc.200700562)the intriguing Pd-catalyzed conversion of 9 to 10. Under mild conditions, it might likely be possible to hydrolyze the vinyl ether to reveal the phenol 11. Another way of looking at this overall transformation would be to consider the ether 10 to be a protected form of the aldehyde 12.
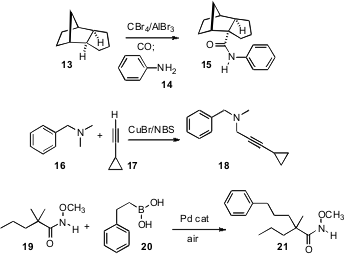
C-H activation can also lead to C-C bond formation. Irena S. Akhrem of the Nesmeyanov Institute, Moscow, described (Tetrahedron Lett. 2008,49, 1399.DOI: 10.1016/j.tetlet.2007.12.070)a hydride-abstraction protocol for three-component coupling of a hydrocarbon 13, an amine 14, and CO, leading to the homologated amide 15. Hua Fu of Tsinghua University, Beijing, showed (J. Org. Chem. 2008, 73, 3961.DOI: 10.1021/jo800279j)that oxidation of an amine 16 led to an intermediate that could be coupled with an alkyne 17 to give the propargylic amine 18.
Products 15 and 18 are the result of sp2 and sp coupling, respectively. C-H functionalization leading to sp3-sp3 coupling is less common. Jin-Quan Yu of Scripps/La Jolla found (J. Am. Chem. Soc. 2008, 130, 7190.DOI: 10.1021/ja801355s)that activation of the N-methoxy amide19 in the presence of the alkyl boronic acid 20 gave smooth coupling, to 21.
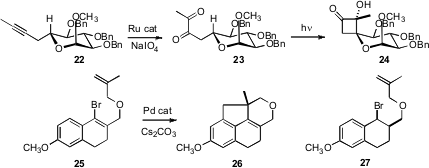
Carbocyclic rings can be constructed using intramolecular C-H functionalization. Antonio J. Herrera and Ernesto Suárez of the CSIC, La Laguna, observed (J. Org. Chem. 2008, 73, 3384.DOI: 10.1021/jo702663w)that 1,2-diketones such as 23, easily prepared by oxidation of the corresponding alkyne, on irradation cyclized with high diastereocontrol to the cyclobutanone, in this case 24. Jayanta K. Ray of the Indian Institute of Technology, Kharagpur found (Tetrahedron Lett. 2008, 49, 851.DOI: 10.1016/j.tetlet.2007.11.172)that exposure of 25 to a Pd catalyst initated a cascade cyclization, delivering the tetracycle 26. It would be interesting to try the same cyclization with the bromide 27. Cyclization might be faster than epimerization and subsequent β-hydride elimination.