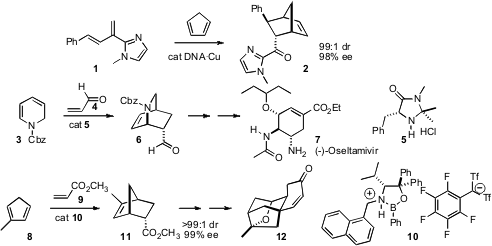
Powerful methods for catalytic, enantioselective intermolecularDiels-Alder reactions have been developed. Ben L. Feringa and Gerard Roelfes of the University of Groningen have shown (Org. PMID:23453497 Lett. 2007, 9, 3647. DOI: 10.1021/ol7015274)that a catalyst prepared by combining salmon testes DNA with a Cu complex directed the absolute sense of the addition of 1 to cyclopentadiene 2. Mukund P. Sibi of North Dakota State University has reported (J. Buy4,6-Dichloropyrimidin-5-amine Am. Chem. Soc. 2007, 129, 395.DOI: 10.1021/ja066425o)related work with achiral pyrazolidinone dienophiles and chiral Cu catalysts. Tohru Fukuyama of the University of Tokyo found (Angew. Chem. Int. Ed. Furan-2,4(3H,5H)-dione site 2007,46, 5734.DOI: 10.1002/anie.200701754)that the MacMillan catalyst 5 was effective at mediating the addition of acrolein 4 to the pyridine-derived diene 3, enabling an enantioselective synthesis of the prominent antiviral (-)-oseltamivir (tamiflu)7. Hisashi Yamamoto of the University of Chicago has demonstrated (J. Am. Chem. Soc. 2007, 129, 9534,DOI: 10.1021/ja073547n and 9536,DOI: 10.1021/ja0735958)that the novel catalyst 10 effected addition of ethyl acrylate 9 to the diene 8, leading to an elegant enantioselective synthesis of the tetracycle 12, the key intermediate in the Nicolaou synthesis of platensimycin.
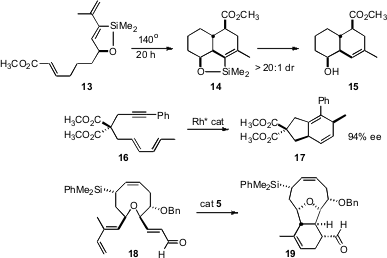
New illustrations of the power of the intramolecular Diels-Alder reaction have been put forward. Demonstrating the influence of a single subsituent on the tether, William R. Roush of Scripps/Florida found (Org. Lett. 2007,9, 2243. DOI: 10.1021/ol070858u)that cyclization of 13 led to the diastereomer 14, complementary to the result observed with an acyclic triene. Ryo Shintani and Tamio Hayashi of Kyoto University have extended (Angew. Chem. Int. Ed. 2007, 46, 7277.DOI: 10.1002/anie.200702586)their studies of chiral diene-based Rh catalysts to the enantioselective cyclization of alkynyl dienes such as 16. Jonathan W. Burton of the University of Oxford and Andrew B. Holmes of the University of Melbourne employed (Chem. Commun. 2007, 3954.DOI: 10.1039/b709322e)the MacMillan catalyst 5 for the cyclization of 18 to 19. It is impressive that ent-5 catalyzed the cyclization of 18 cleanly into the diastereomer of 19 in which both of the newly-created stereogenic centers were inverted.
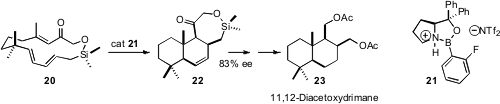
Transannular intramolecular Diels-Alder (TADA) cyclizations have been widely employed. Although a great deal has been learned about relative stereocontrol, little progress had been made on asymmetric catalysis of the cyclization of prochiral trienes such as 20. Eric N. Jacobsen of Harvard University has now found (Science 2007, 317, 1736.DOI: 10.1126/science.1146939)that the o-fluoro complex 21 served effectively. The power of this approach was illustrated by the conversion of the adduct 22 into the natural product 11,12-diacetoxydrimane 23.