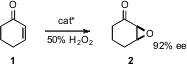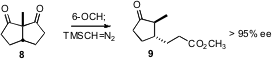Benjamin List of the Max Planck Institute, Mülheim devised (J. PMID:24293312 Am. Chem. Soc. 2008, 130, 6070.DOI: 10.1021/ja801181u)a chiral primary amine salt that catalyzed the enantioselectiveepoxidation of cyclohexenone 1. Larger ring and alkyl-substituted enones are also epoxidized with high ee.
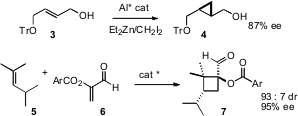
Three- and four-membered rings are versatile intermediates for further transformation. 6-Bromo-2(1H)-quinolinone structure Tsutomu Katsuki of Kyushu University developed (Angew. 1403257-80-6 Purity Chem. Int. Ed. 2008, 47, 2450.DOI: 10.1002/anie.200705641)an elegant Al(salalen) catalyst for the enantioselectiveSimmons-Smith cyclopropanation of allylic alcohols such as 3. Kazuaki Ishihara of Nagoya University found (J. Am. Chem. Soc. 2007, 129, 8930.DOI: 10.1021/ja073435w)chiral amine salts that effected enantioselective 2+2 cycloaddition of α-acyloxyacroleins such as 5 to alkenes to give the cyclobutane7 with high enantio- and diastereocontrol.
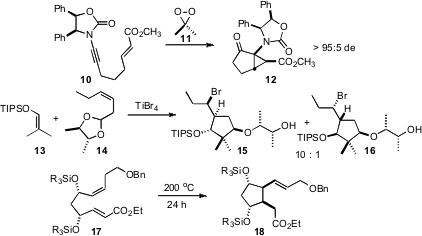
Gideon Grogan of the University of York overexpressed (Adv. Synth. Catal. 2008, 349, 916.DOI: 10.1002/adsc.200600468)the enzyme 6-oxocamphor hydrolase in E. coli. The 6-OCH so prepared converted prochiral diketones such as 8 to the cyclopentane 9 in high ee. Richard P. Hsung of the University of Wisconsin found (Org. Lett. 2008, 10, 661DOI: 10.1021/ol703083k)that the carbene produced by oxidation of the ynamide 10 cyclized to 11 with high de. Teck-Peng Loh of Nanyang Technological University extended (J. Am. Chem. Soc. 2008, 130, 7194.DOI: 10.1021/ja801488z)butane-2,3-diol directed cyclization to the preparation of the cyclopentane 15. Note that sidechain relative configuration is also controlled. We established (J. Org. Chem. 2008, 73, 3467. DOI: 10.1021/jo702600v)that the thermal ene reaction of 17 delivered the tetrasubstituted cyclopentane 18 as a single diastereomer.
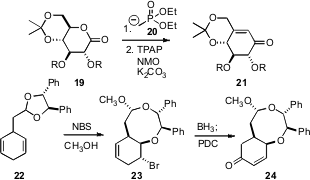
Tony K. M. Shing of the Chinese University of Hong Kong devised (J. Org. Chem. 2007, 72, 6610.DOI: 10.1021/jo0709697)a simple protocol for the conversion of carbohydrate-derived lactones such as 19 to the highly-substituted, enantiomerically-purecyclohexenone 21. Hiromichi Fujioka and Yasuyuki Kita of Osaka University established (Org. Lett. 2007, 9, 5605. DOI: 10.1021/ol702530b)a chiral diol-mediated conversion of the cyclohexadiene 22 to the diastereomerically pure cyclohexenone 24.
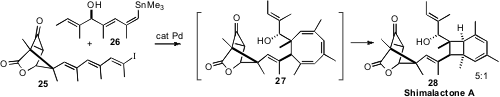
Dirk Trauner, now of the University of Munich, reported (Org. Lett. 2008, 10, 149.DOI: 10.1021/ol702806v)an elegant assembly of the neuritogenic polyketide shimalactone A (28). As anticipated, the polyene prepared byStille coupling of the iodide 25 with the stannane 26 underwent 8 π cyclization to 27, that then spontaneously underwent 8 π cyclization to 28, with substantial diastereocontrol.