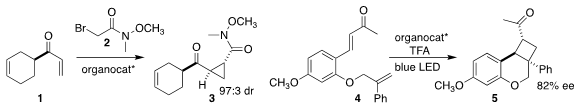
Gullapalli Kumaraswamy of the Indian Institute of Chemical Technology
assembled the cyclopropane 3 by the (DHQD)
Pyr-mediated addition of the bromo amide 2 to the enone 1
(Tetrahedron 2021, 87, 132110.
DOI: 10.1016/j.tet.2021.132110).
José Alemán of the Universidad Autónoma de Madrid showed that a bis-naphthyl-1,2-diamine
mediated the photocyclization of the diene 4, leading to the
cyclobutane 5
(Chem. Commun. PMID:26780211 2021, 57, 3046.
DOI: 10.1039/D1CC00035G).
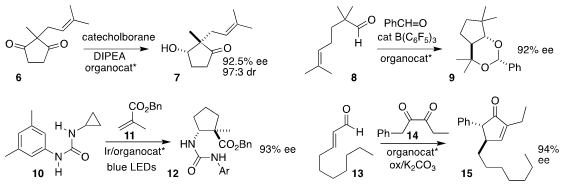
Fu-She Han of the Changchun Institute of Applied Chemistry used a P-stereogenic phosphinamide
to direct the reduction of the prochiral diketone 6 to the keto alcohol 7
(J. 1359656-11-3 Price 1-Ethynyl-3,5-dimethylbenzene structure Am. Chem. Soc. 2021, 143, 2994.
DOI: 10.1021/jacs.1c00277).
Manabu Hatano of Kobe Pharmaceutical University and Kazuaki Ishihara of Nagoya University used a
BINOL-derived phosphoric acid to mediate the cyclization of the aldehyde 8 to
the protected 1,3-diol 9
(ACS Catal. 2021, 11, 6121.
DOI: 10.1021/acscatal.1c01242).
Takashi Ooi, also of
Nagoya University, demonstrated that a racemic Ir photocatalyst complexed with a
BINOL-derived chiral borate effectively catalyzed the opening of the
cyclopropylamine 10 and subsequent addition to the methacrylate 11, leading to
the cyclopentanecarboxylate 12
(Org. Biomol. Chem. 2021, 19, 1744.
DOI: 10.1039/D1OB00126D).
Shuang Yang and Xinqiang Fang of the University of the Chinese Academy of Sciences, Fuzhou,
and Jinggong Liu and Bolai Chen of the Guangdong Provincial Hospital of
Traditional Chinese Medicine employed an NHC catalyst to combine the aldehyde 13 with the 1,2-diketone
14, to give the
cyclopentenone 15
(Org. Lett. 2021, 23, 3403.
DOI: 10.1021/acs.orglett.1c00870).
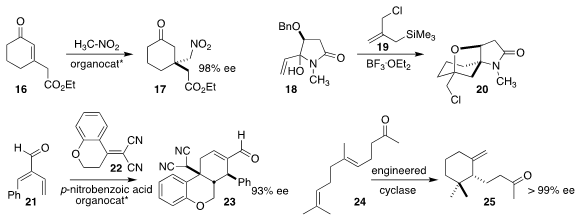
Jian-Liang Ye and Pei-Qiang Huang of Xiamen University used a
thiourea-primary amine catalyst to effect the conjugate addition of nitromethane
to the cyclohexenone 16, leading to the ester 17
(Tetrahedron 2021, 84, 132005.
DOI: 10.1016/j.tet.2021.132005).
Stephen G. Pyne of the University of Wollongong assembled the
cyclohexane 20 by
combining the allyl silane 19 with the tertiary alcohol 18
(Org. Biomol. Chem. 2021, 19, 259.
DOI: 10.1039/D0OB02075C).
Ying-Chun Chen of Sichuan University showed that a modified
Jørgenson-Hayashi catalyst effectively directed the addition of the bis nitrile 22 to
the unsaturated aldehyde 21, leading to the cyclohexenecarboxaldehyde 23
(Org. Biomol. Chem. 2021, 19, 151.
DOI: 10.1039/D0OB02068K).
Bernhard Hauer of the University of Stuttgart devised a modified terpene cyclase
that converted the ketone 24 to the cyclohexane 25
(Angew. Chem. Int. Ed. 2021, 60, 13251.
DOI: 10.1002/anie.202101228).
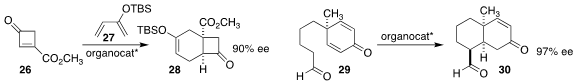
Ping Lu of Fudan University used a chiral oxazaborolidinium ion to catayze
the Diels-Alder cycloaddition of the
cyclobutenone 26 to the diene 27, leading
to the bicyclic ketone 28
(Angew. Chem. Int. Ed. 2021, 60, 4609.
DOI: 10.1002/anie.202014308).
Shuanhu Gao of East China Normal University showed that the Jørgenson-Hayashi catalyst cyclized
the prochiral cyclohexadienone 29 to the bicyclic enone 30
(Chem. Sci. 2021, 12, 4747.
DOI: 10.1039/D0SC07089K).
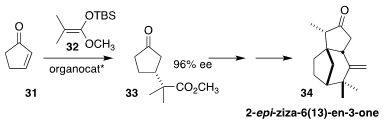
Vetiver oil, obtained by hydrodistillation of the dried roots of the tufted
perennial grass Chrysopogon zizanioides, is omnipresent in perfumery. Philip
Kraft of Givaudan and Benjamin List of the Max-Planck-Institut für
Kohlenforschung demonstrated by total synthesis, beginning with the
imidodiphosphorimidate-catalyzed enantioselective conjugate addition of the
ketene silyl acetal 32 to cyclopentenone 31 to give 33, that the lead fragrance
contributor of the oil is 2-epi-ziza-6(13)-en-3-one (34), a minor component
(Angew. Chem. Int. Ed. 2021, 60, 5666.
DOI: 10.1002/anie.202014609).