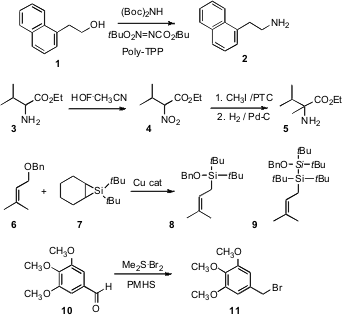
Jeffrey C. Pelletier of Wyeth Research, Collegeville, PA has developed (Tetrahedron Lett. 2007, 48, 7745. DOI: 10.1016/j.tetlet.2007.09.034)a easy work-up Mitsunobu procedure for the conversion of a primary alcohol such as 1 to the corresponding primary amine 2. Shlomo Rozen of Tel-Aviv University has taken advantage (J. PMID:23554582 150852-73-6 Order Org. Chem. 2007, 72, 6500. DOI: 10.1021/jo0709450)of his own method for oxidation of a primary amine to the nitro compound to effect net conversion of an amino ester 3 to the alkylated amino ester 5. Note that the free amine of 3 or 5 would react immediately with methyl iodide. Keith A. Woerpel of the University of California, Irvine has uncovered (J. Am. Chem. Soc. 2007, 129, 12602. DOI: 10.1021/ja073758s)a Cu catalyst that, with7, effected direct conversion of silyl ethers such as 6 to the allyl silane 8. Pyrazine-2,3-diamine Chemscene An Ag catalyst gave 9, which also shows allyl silane reactivity. Biswanath Das of the Indian Institute of Chemical Technology, Hyderabad has established (Tetrahedron Lett. 2007, 48, 6681. DOI: 10.1016/j.tetlet.2007.07.125)a compact procedure for the direct conversion of an aromatic aldehyde such as 10 to the benzylic halide 11. This will be especially useful for directly generating benzylic halides that are particularly reactive.
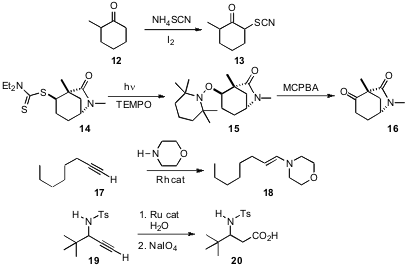
α-Sulfinylation of ketones often requires intial generation of the enolate. J. S. Yadav, also of the Indian Institute of Chemical Technology, Hyderabad, has devised (Tetrahedron Lett. 2007, 48, 5243.DOI: 10.1016/j.tetlet.2007.05.143)an oxidative protocol for installing sulfur adjacent to a ketone. In a related development, Richard S. Grainger of the University of Birmingham has established (Angew. Chem. Int. Ed. 2007, 46, 5377. DOI: 10.1002/anie.200701055)a simple procedure for the conversion of thio esters such as 14 to the corresponding ketone 16. Yoshiya Fukumoto of Osaka University has shown (J. Am. Chem. Soc. 2007, 129, 13792.DOI: 10.1021/ja075484e)that a terminal alkyne17 can be directly converted into the enamine 18 by Rh-catalyzed addition of a secondary amine. Lukas Hintermann and Carsten Bolm of RWTH Aachen have found (J. Org. Chem. 2007, 72, 5704. DOI: 10.1021/jo070745o)that inclusion of water gave the aldehyde, which could be oxidized with the residual Ru catalyst to the acid.
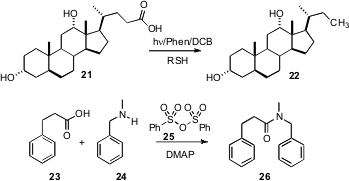
Yasuharu Yoshimi and Minoru Hatanaka of the University of Fukui have described (Chem. Comm. 2007, 5244.DOI: 10.1039/b714526h)a convenient procedure for the reductivedecarboxylation of acids such as 21 to the corresponding alkane 22. Teruaki Mukaiyama of the Kitasato Institute, Tokyo has developed (Chemistry Lett. 2007, 36, 1456.DOI: 10.1246/cl.2007.1456)a simple protocol for the activation of carboxylic acids for amide formation. DMAP-mediated coupling of the acid with 25 gave the mixed anhydride, which combined efficiently with the amine 24 to give the amide 26.