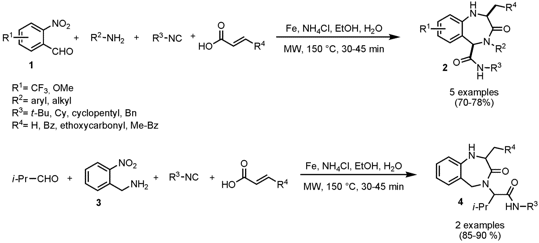
The group of Peter Andreana from Wayne State University, Michigan, has developed a one-pot two-step synthesis of regiochemically differentiated 1,4-benzodiazepin-3-ones (Org. PMID:24633055 Lett. 2008, 10, 4541. (6S)-Hexahydro-1,4-oxazepin-6-ol In stock DOI: 10.1021/ol801841m). The first step involves the Ugi four-component coupling reaction to form α-acylaminoamides which are subsequently converted to benzodiazepines by a reductive (NO2 → NH2) aza-Michael cyclization employing Fe(0)/NH4Cl in aqueous media as reducing agent. With bifunctional o-nitrobenzaldehyde 1 exclusively C2, N4, C5-substituted benzodiazepines 2 are obtained whereas with o-nitrobenzylamine 3 the C2, N4-substituted derivatives 4 are formed. Price of 4-Nitrobenzenethiol The reduction reaction gave higher yields in shorter times compared to more conventional reactions performed in a sealed-tube with oil-bath heating. It has to be noted that at higher temperatures either decomposition or a 6-exo aza-Michael cyclization to form 2,5-diketopiperazines occurred.
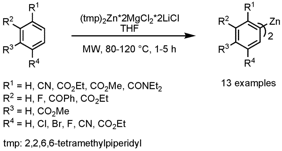
High-Temperature Zincation of Functionalized (Het)Aromatics
Paul Knochel and Stefan Wunderlich from Ludwig-Maximilians-Universität, Munich, have reported on the direct zincation of highly functionalized aryl and heteroaryl derivatives using the complex base (tmp)2Zn•2MgCl2•2LiCl (Org. Lett. 2008, 10, 4705.DOI: 10.1021/ol802118e). With microwave heating, a tremendous time saving could be achieved − e.g. for derivatives with R1 = CO2Et, R2 = R3 = H, R4 = Cl, Br the reaction time could be reduced from 110 h at 25 °C to 2 h at 80 °C. Interestingly, for substrates with R1 = CO2Et, CONEt2, R2 = R3 = R4 = H, microwave heating is crucial since under oil-bath heating at the same temperature a very low yield is obtained (10-20% vs. >90%). Importantly, sensitive functional groups are well tolerated under the reaction conditions. This zincation protocol was further successfully applied to heterocyclic systems such as pyridines, benzothiophene or benzofuran. In a post-derivatization step, the bis-zincated species were further transformed via Cu-mediated orPd-catalyzed reactions.
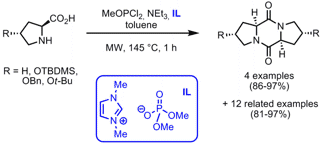
Synthesis of Symmetrical and Unsymmetrical 2,5-Diketopiperazines
The phosphorous-promoted coupling of unprotected amino acids toward the one-step synthesis of 2,5-diketopiperazines was disclosed by Stefan Bräse and his group from the Universität Karlsruhe, Germany (Eur. J. Org. Chem. 2008, ASAP.DOI: 10.1002/ejoc.200800605). Symmetrical and unsymmetrical 2,5-diketopiperazines are generated by the condensation of amino acids with methyldichlorophosphite in toluene. The ionic liquid (IL) 1,3-dimethylimidazolium dimethyl phosphate needs to be used as a heating aid in order to reach the 145°C. Unsymmetrical derivatives are obtained by the combination of proline, sarcosine, indoline- and octahydroindolecarboxylic acids without the formation of any by-products (1.2 equiv excess of one amino acid is crucial in this case). Advantages of this protocol are excellent yields, complete retention of the stereochemistry, tolerance of base-stable protecting groups and a simple work-up procedure (only filtration).
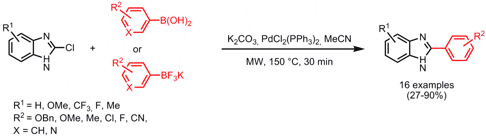
Suzuki-Miyaura Coupling of Unprotected 2-Chlorobenzimidazoles
Brad Savall and Jill Fontimayor from Johnson & Johnson Pharmaceutical Research & Development, San Diego, have synthesized a series of 2-arylbenzimidazoles via Suzuki-Miyaura couplings of unprotected 2-chlorobenzimidazoles (Tetrahedron Lett. 2008, 49, 6667.DOI: 10.1016/j.tetlet.2008.09.043). Higher yields could be achieved when switching from arylboronic acids to aryltrifluoroborate salts as coupling partners. To overcome the problem with homocoupling of pyridylboronic acids, a change in the catalyst system to PdCl2(dppf)•CH2Cl2 and the use of aryltrifluoroborate salts was advantageous.
.