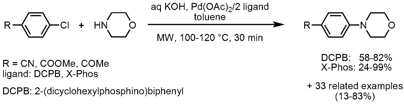
A small library of 2,4(1H,3H)-quinazolinediones (X = O) and 2-thioxoquinazolines (X = S) was prepared via the reaction of substituted methyl anthranilate with diverse iso(thio)cyanates without the addition of catalyst, ligand or base by Hong Liu and co-workers from the Chinese Academy of Sciences in Shanghai (J. Comb.Chem. 2008, 10, 484. DOI: 10.1021/cc800040z). PMID:27102143 Aryl-substituted iso(thio)cyanates proved to give higher product yields compared to aliphatic ones. In addition, the products could be easily isolated by precipitation and filtration due to the employed DMSO/H2O solvent mixture. 2-Cyclopentenone custom synthesis
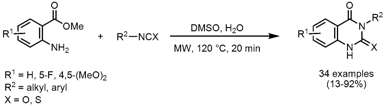
N-Arylation of Amines
Hong Lui and co-workers from the Shanghai Institute of Biological Sciences have reported on the copper-mediated N-arylation of amines with boronic acids (J. Comb. Chem. 2008, 10, 358. DOI: 10.1021/cc8000053). 1627973-06-1 Order The coupling of various amines with differently substituted boronic acids under mild conditions and in the absence of a Pd catalyst led to a small library of 21 arylated products. Importantly, in addition to anilines this protocol is applicable for primary and secondary alkyl amines and nitrogen containing heterocycles where a reduced reaction time of 15 min is sufficient to obtain the coupled products in 48-96% yield.
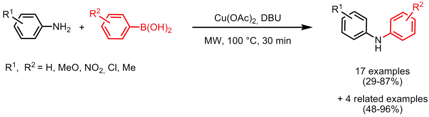
Pd-Catalyzed Amination of Aryl chlorides
Gitte Van Baelen and Bert Maes from the University of Antwerp, Belgium, have developed a protocol that allows selective hydrolysis of nitriles to amides or avoids hydrolysis of esters and nitriles by employing a toluene/concentrated aqueous KOH two phase system under microwave conditions (Tetrahedron 2008, 64, 5604. DOI: 10.1016/j.tet.2008.03.028). Selective hydrolysis can be achieved by the use of a phase transfer catalyst (PTC) whereas no hydrolysis occurs in the absence of a PTC under otherwise identical conditions. By employing the reaction conditions discovered in this study for the Pd-catalyzed amination of aryl chlorides bearing sensitive functional groups such as nitriles or esters with aliphatic amines, successful coupling to the corresponding products without the occurrence of hydrolysis could be achieved. When primary amines are employed JohnPhos proved to be a superior ligand. Anilines can be coupled as well, although the yields are rather low. Heteroaromatics are also suitable substrates for this coupling protocol. Importantly, the key factor for successful couplings is the ratio of toluene and concentrated KOH solution: when 1 mL toluene is used compared to 0.5 mL nearly no product formation was obtained.
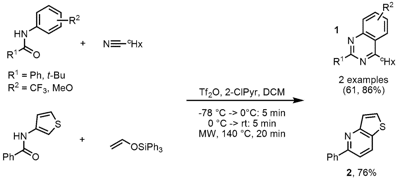
Synthesis of Azaheterocycles
A comparison study of oil-bath and microwave heating in the synthesis of quinazolines 1 and pyridine 2 was performed by Matthew Hill and Mohammad Movassaghi from the Massachusets Institute of Technology (Tetrahedron Lett. 2008, 49, 4286. DOI: 10.1016/j.tetlet.2008.04.143). Microwave irradiation proved to be beneficial for electron-deficient and sterically hindered amides or for weak nucleophiles. Improved yields could be achieved with microwave heating at 140 °C for 20 min compared to oil-bath heating under otherwise identical conditions. At lower temperatures of 75 °C no difference in the reaction outcome was observed, however 30-70% lower yields were obtained.
.