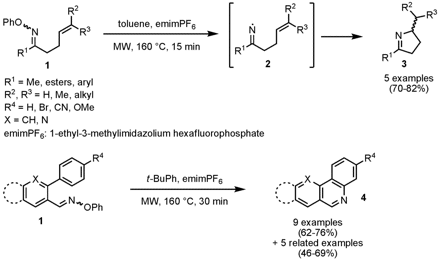
John Walton and co-workers from the University of St. Andrews, UK, have reported on the use of O-phenyl oxime ethers as precursors for iminyl radicals en route for N-heterocycle synthesis (J. Org. Chem. 2008, 73, 5558. DOI: 10.1021/jo800847h). Microwave irradiation was employed for the thermolysis of functionalized O-phenyl oximes 1 generating the iminyl radicals 2 followed by cyclization to the corresponding heterocycles. For the synthesis of dihydropyrroles 3, toluene is used as solvent whereas a non-H-atom donor solvent such as t-butylbenzene has to be employed for cyclizations onto aromatic radical acceptors for the synthesis of quinolines, phenanthridines (4, X = CH), benzonaphthiridines (4, X = N) and indolopyridines. N-Methyl-3-phenylpropan-1-amine site For both protocols the ionic liquid emimPF6 needs to be used in order to reach the 160 °C reaction temperature. PMID:25027343 22112-84-1 Purity With this method, no initiator is necessary and in combination withmicrowave heating, cleaner product mixtures and shortened reaction times can be achieved.
Stereoselective Synthesis of Z-Dienes
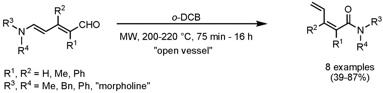
Christopher Vanderwal and his group from the University of California, Irvine, have discovered a new method for the stereoselective synthesis of Z-α,β,γ,δ-unsaturated amides (J. Am. Chem. Soc. 2008, 130, 7560. DOI: 10.1021/ja8028125). Zincke aldehydes (5-amino-2,4-pentadienals) rearrange thermally to the corresponding α,β,γ,δ-unsaturated amides via a pericyclic cascade process. ExcellentZ-selectivity (>20:1) is observed in most of the cases. Preliminary experiments indicated that the rearrangement rates could be increased by addition of small amounts of camphorsulfonic acid.
One-Pot Two-Step Synthesis of Benzoxazoles
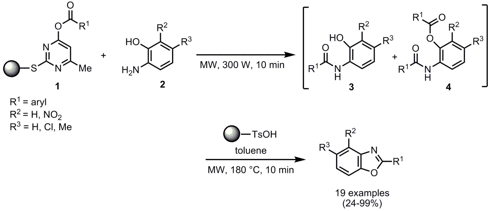
The synthesis of highly substitutedbenzoxazoles employing solid-supported reagents was developed by Maurizio Botta and his group from Università di Siena, Italy (Tetrahedron Lett. 2008, 49, 4464.DOI: 10.1016/j.tetlet.2008.05.059). In the first step solid supported reagents 1 were reacted neat with 2-aminophenols 2 to give the uncyclized mono- and diacylated intermediates 3 and 4. Subsequent addition of toluene and polymer-bound p-toluenesulfonic acid to catalyze the cyclodehydration and irradiation at 180 °C for 10 min gave the benzoxazole derivatives in moderate to excellent yields. Importantly, the products are obtained in high purity by final filtration from the polymer-supported reagents. By combining a parallel synthesizer for evaporation and filtration/washing steps with microwave heating, a small library was produced in a short time frame which renders the protocol amenable for automation.
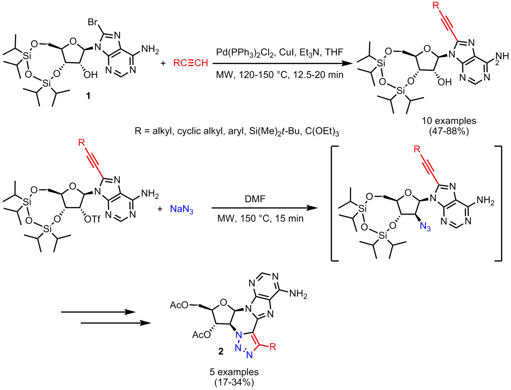
Synthesis of Cyclonucleosides
Adenosine-based nucleosides 2 which exhibit fluorescence in the visible range and that are bridged via a 1,2,3-triazole moiety between the purine base and the ribose were prepared by Morten Grøtli and co-workers from the University of Gothenburg, Sweden (Tetrahedron 2008, 64, 7151.DOI: 10.1016/j.tet.2008.05.098). For installing the 8-alkynyl functionality for the cyclization precursor a Sonogashira reaction between 8-bromoadenosine derivative 1 and various terminal alkynes was performed under microwave heating. After triflation of the 2’-OH group, the 2’-azide functionality was introduced by nucleophilic displacement. The displacement and subsequent intramolecular cyclization via Huisgen [3+2]-cycloaddition of the alkyne and the 2’-azide was again accomplished under microwave heating. Since these conditions proved to be incompatible with the silyl protecting groups, complete deprotection and subsequent protection with acetic anhydride due to isolation problems had to be performed for obtaining the cyclonucleosides 2 in moderate yields. For both synthesis steps better results regarding conversion were achieved with microwave heating.
.