Reaction with an enantiomerically-pure epoxide is an efficient way to construct a molecule incorporating an enantiomerically-pure oxygenated stereogenic center. The Jacobsen hydrolytic resolution has made such enantiomerically-pure epoxides readily available from the corresponding racemates. Christopher Jones and Marcus Weck of the Georgia Institute of Technology have now (J. Am. Chem. Soc. 2007, 129, 1105. DOI: 10.1021/ja0641406)developed an oligomeric salen complex that effects the enantioselective hydrolysis at remarkably low catalyst loading. PMID:23991096
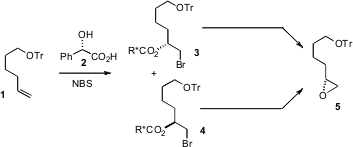
Any such approach depends on monitoring the progress of the hydrolysis, usually by chiral GC or HPLC. In a complementary approach, we (J. Org. Chem. 2007, 72, 431. 5-Bromo-1,3,4-thiadiazole-2-carbaldehyde Purity DOI: 10.1021/jo061818r)have found that on exposure to NBS and the inexpensive mandelic acid 2, a terminal alkene such as 1 was converted into the two bromomandelates 3 and 4. These were readily separated by column chromatography. Individually, 3 and 4 can each be carried on the same enantiomer of the epoxide 5. Formula of N-Boc-PEG2-bromide As 3 and 4 are directly enantiomerically pure, epoxide 5 of high ee can be prepared reliably without intermediate monitoring by chiral GC or HPLC.
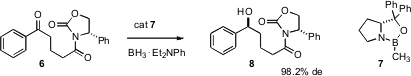
Another way to incorporate an enantiomerically-pure oxygenated stereogenic center into a molecule is the enantioface-selective addition of hydride to a ketone such as6. Alain Burgos and his team at PPG-SIPSY in France have described (Tetrahedron Lett. 2007, 48, 2123. DOI: 10.1016/j.tetlet.2007.01.118)a NaBH4-based protocol for taking the Itsuno-Corey reduction to industrial scale.
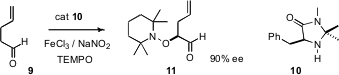
In the past, aldehydes have been efficiently α-oxygenated using two-electron chemistry. Mukund P. Sibi of North Dakota State University has recently (J. Am. Chem. Soc. 2007, 129, 4124.DOI: 10.1021/ja069245n)described a novel one-electron alternative. The organocatalyst 10 formed an imine with the aldehyde. One-electron oxidation led to an α-radical, which was trapped by the stable free radicalTEMPO to give, after hydrolysis, the α-oxygenated aldehyde 11.
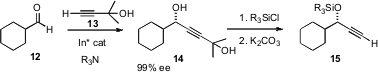
High ee oxygenated secondary centers can also be prepared by homologation of aldehydes. Optimization of the enantioselective addition of the inexpensive acetylene surrogate 13 was recently reported (Chem. Commun. 2007, 948.DOI: 10.1039/b614958h)by Masakatsu Shibasaki of the University of Tokyo. Note that the free alcohol of 13 does not need to be protected.
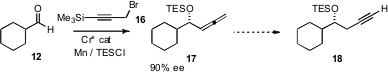
A protocol for enantioselective allenylation was recently developed (J. Am. Chem. Soc. 2007, 129, 496.DOI: 10.1021/ja0679578)by Hisashi Yamamoto of the University of Chicago. The conversion of 17 to 18 should be straightforward, leading to net enantioselective propargylation.
The enantioselective homologation of ketones, in particular methyl ketones, can also be accomplished. In this procedure described (J. Am. Chem. Soc. 2007, 129, 7439. DOI: 10.1021/ja071512h)by Professor Shibasaki and Motomu Kanai, also of the University of Tokyo, the alkyl group from the Zn was incorporated into the product. Alternative dialkyl zincs worked as well.