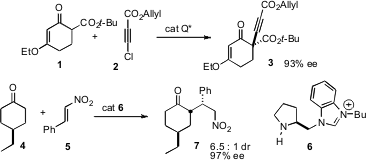
One of the more powerful routes to enantiomerically-purecarbocycles is the desymmetrization of a prochiral ring. Karl Anker Jørgensen of Aarhus University has found (J. Am. Chem. Soc. 2007, 129, 441. DOI: 10.1021/ja067289q)that many cyclic β-ketoesters, including the vinylogous carbonate 1, can be homologated with 2 to the corresponding alkyne 3, in high ee. Sanzhong Luo of the Chinese Academy of Sciences, Beijing, and Jin-Pei Cheng, of the Chinese Academy of Sciences and Nankai University, have shown (J. 72607-53-5 manufacturer Org. Chem. 2007, 72, 9350. DOI: 10.1021/jo7020357)that the catalyst 6 mediated the selective addition of 4-substituted cyclohexanones such as 4 to the nitroalkene 5, establishing three new stereogenic centers.
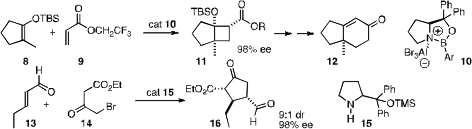
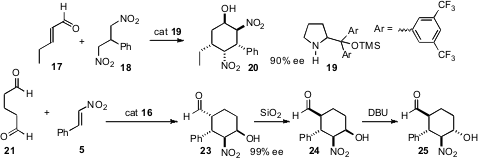
Organocatalysts, alone or complexed with activating metals, have also been used to effect enantioselective ring construction. E. J. Corey of Harvard University has established (J. Am. Chem. PMID:23916866 5-Methoxypicolinimidamide hydrochloride Chemical name Soc. 2007, 129, 12686. DOI: 10.1021/ja0765262)that the proline-derived complex 10 will mediate the 2+2 addition of a cyclic enol ether with an acrylate to give the cyclobutane 11. Further elaboration led to the cyclohexenone 12. Armando Córdova of Stockholm University has described (Tetrahedron Lett. 2007, 48, 5835.DOI: 10.1016/j.tetlet.2007.06.070) a novel route to cyclopentanones such as 16, via tandem conjugate addition/intramolecular alkylation. Professor Jørgensen has reported (Angew. Chem. Int. Ed. 2007, 46, 9202.DOI: 10.1002/anie.200704454)the double addition of 18 to the unsaturated aldehyde 17 to give 20. Earlier last year, Yujiro Hayashi of the Tokyo University of Science had shown (Angew. Chem. Int. Ed. 2007, 46, 4922.DOI: 10.1002/anie.200700909)that the double addition of the inexpensive 21 to 5 could, depending on conditions, be directed selectively to 22, 23, or 24.
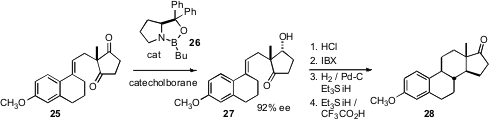
As illustrated by the conversion of 8 to 13, organocatalysis can be used to effect the enantioselective construction of polycarbocyclic products. The initial ring prepared in enantiomerically-pure form by organocatalysis can also set the chirality of a polycyclic system. Professor Corey has reported (J. Am. Chem. Soc. 2007, 129, 10346.DOI: 10.1021/ja0742434)that Itsuno-Corey reduction of the prochiral diketone 25 led to the ketone 27. Cyclization followed by oxidation and reduction then delivered estrone methyl ether 28.
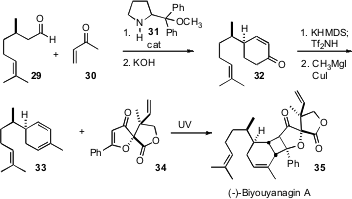
Professor Hayashi, Professor Jørgensen and Samuel H. Gellman of the University of Wisconsin had established ( 2006, July 24) that an aldehyde could be added to a vinyl ketone in high ee. Building on these results, K. C. Nicolaou of Scripps/La Jolla has found (Angew. Chem. Int. Ed. 2007, 46, 4708.DOI: 10.1002/anie.200701552)that the initial Michael adduct from the condensation of (R)-citronellal 29 with methyl vinyl ketone 30 can be converted into the Robinson annulation product 32 without epimerization at the gamma position. This led to 33, cycloaddition of which with34 delivered (-)-biyouyanagin 35.
2006, July 24) that an aldehyde could be added to a vinyl ketone in high ee. Building on these results, K. C. Nicolaou of Scripps/La Jolla has found (Angew. Chem. Int. Ed. 2007, 46, 4708.DOI: 10.1002/anie.200701552)that the initial Michael adduct from the condensation of (R)-citronellal 29 with methyl vinyl ketone 30 can be converted into the Robinson annulation product 32 without epimerization at the gamma position. This led to 33, cycloaddition of which with34 delivered (-)-biyouyanagin 35.