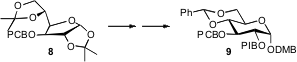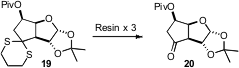Benzyl esters are easily deprotected by hydrogenolysis. It is often observed, however, as exemplified by the conversion of 1 to 2 reported (Adv. Synth. Catal. 6-Bromo-4(1H)-cinnolinone Chemical name 2008, 350, 406.DOI: 10.1002/adsc.200700571)by Hironao Sajiki of Gifu Pharmacutical University, that alkene hydrogenation can be carried out selectively. Fernando Albericio of the University of Barcelona has developed (Tetrahedron Lett. 2008, 49, 3304.DOI: 10.1016/j.tetlet.2008.03.074)a family of thiophene-based esters 3 that can be removed with acid in the presence of t-butyl esters, and that are stable to the removal of FMOC groups. Vassiliki Theodorou of the University of Ioannina has found (Tetrahedron Lett. 2008, 49, 8230.DOI: 10.1016/j.tetlet.2007.09.074)that esters were rapidly saponified by methanolic NaOH in solvent CH2Cl2. (S)-2-Methoxy-1-phenylethan-1-amine In stock
Specific oligosaccharide synthesis depends heavily on the use of orthogonal methods for alcohol protection and deprotection. This is illustrated by the work (J. PMID:24633055 Org. Chem. 2008, 73, 1008.DOI: 10.1021/jo702032c)of Carolyn R. Bertozzi of the University of California, Berkeley, who deployed p-methoxybenzyl (PMB), 3,4-dimethoxybenzyl (DMB), p-chlorobenzyl (PCB) and p-iodobenzyl (PIB) ethers to enable construction of a disaccharide, by way of 9. Piers R. J. Gaffney of Imperial College London has reported (Tetrahedron Lett. 2008, 49, 1836.DOI: 10.1016/j.tetlet.2008.01.039)a practical preparation of the ether 11, that should make this symmetrical protecting group more readily available. George W. J. Fleet of the University of Oxford and Sigthur Petursson of the University of Akureyri have found (Tetrahedron Lett. 2008, 49, 2196.DOI: 10.1016/j.tetlet.2008.02.042)that diphenyl diazomethane 14, easily prepared from benzophenone, reacted under neutral conditions with primary, secondary and tertiary alcohols to form the benzyhydryl ethers. Harsh conditions have often been employed to remove aryl methyl ethers such as 16. Wei Wang of the University of New Mexico and Wenhu Duan of the Shanghai Institute of Materia Medica have developed (Tetrahedron Lett. 2008, 49, 4054.DOI: 10.1016/j.tetlet.2008.04.070)a simple protocol to effect this transformation, by heating the ether to reflux in DMF in the presence of iodocyclohexane 17.
Dithianes such as 19 have often been deprotected with stoichiometric heavy metals. Andreas Kirschning of Leibniz Universität Hannover has devised (J. Org. Chem. 2008, 73, 2018.DOI: 10.1021/jo7025146)a set of three anionic resins, charged, respectively, with I(O2CCF3)2–, HCO3–, and S2O3–. Exposure of 19 to the three resins in sequence delivered the very sensitive ketone 20.
Clotilde Ferroud of the Conservatoire National des Arts et Métiers, Paris has established (Tetrahedron Lett. 2008, 49, 3004.DOI: 10.1016/j.tetlet.2008.02.170)microwave conditions for the direct acetylation of an amine such as 21 with vinyl acetate 22. Samad Khaksar of the Islamic Azad University, Iran has found (Tetrahedron Lett. 2008, 49, 3527.DOI: 10.1016/j.tetlet.2008.03.138)that thiourea 25 catalyzed the protection of an amine 24 with Boc2O under similarly mild conditions. Darren J. Dixon of the University of Manchester employed (Chem. Commun. 2008, 2474.DOI: 10.1039/b802447b)the protocol developed by Romo to convert the sulfonamide 27 to the trifluoracetate 28. Uno Mäeorg of the University of Tartu showed (Tetrahedron Lett. 2008, 49, 1373.DOI: 10.1016/j.tetlet.2007.12.098)that sulfonamides can also be deprotected with the inexpensive mischmetal in the presence of TiCl4.