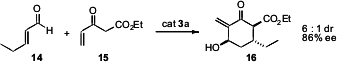Armando Córdova of Stockholm University has found (Tetrahedron Lett. 2008, 49, 4209. DOI: 10.1016/j.tetlet.2008.04.162)that the organocatalyst 3a effected enantioselective conjugate addition of bromonitromethane 2 to the α,β-unsaturated aldehyde 1, to give the cyclopropane 4 as a ~ 1:1 diastereomeric mixture, both in high ee. Tomislav Rovis of Colorado State University has published (J. Org. PMID:24733396 Chem. 2008, 73, 2033. DOI: 10.1021/jo702313f)a detailed account of his development of catalysts such as 6, that effected enantioselective cyclization of 5 to 7 with excellent ee. Karl Anker Jørgensen of Aarhus University has employed (J. Am. BuyFmoc-NH-PEG4-CH2CH2COOH 6-(Diphenylphosphino)-2,2′-bipyridine Formula Chem. Soc. 2008, 130, 4897. DOI: 10.1021/ja710689c)chiral quaternary salts derived from quinine that mediated the enantioselective addition of prochiral rings such as 8 to the allenoate ester9 to give 10 with high ee.
Organocatalysts have also been used to prepare more highly substituted cyclohexane derivatives. Guofu Zhong of Nanyang Technological University used (Org. Lett. 2008, 10, 2437. DOI: 10.1021/ol8007183)a quinine-derived secondary amine to catalyze the Michael addition of 12 to 11 followed by intramolecularaldol (Henry) reaction, to give13. When Professor Jørgensen attempted (Angew. Chem. Int. Ed. 2008, 47, 121. DOI: 10.1002/anie.200704076)the related addition of 14 and 15 using catalyst 3a, he did not observe the expected Michael-Michael sequence. Rather, the initial Michael addition was followed by a Morita-Baylis-Hillman condensation, to give 16. The β-keto ester 16 existed primarily in its enol form.
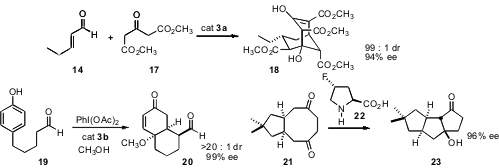
Organocatalysts can also be used to prepare polycyclic systems. Professor Jørgensen has found (Chem. Commun. 2008, 3016. DOI: 10.1039/b806418k)that condensation of 14 with acetone dicarboxylate 17, again using catalyst 3a, gave the bicyclic β-keto ester 18. Matthew J. Gaunt of the University of Cambridge observed (J. Am. Chem. Soc. 2008, 130, 404.DOI: 10.1021/ja077457u)that for the cyclization of 19, catalyst 3b was superior to catalyst 3a. The power of desymmetrization of prochiral intermediates was illustrated by the report (J. Am. Chem. Soc. 2008, 130, 6737.DOI: 10.1021/ja8024164)from Benjamin List of the Max-Planck-Institute, Mülheim of the cyclization of 21 to 23.
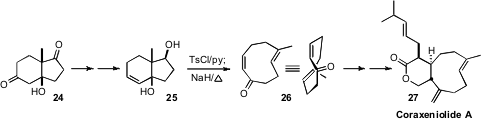
Organocatalysts can also be used to prepare larger rings. The Hajos-Parrish condensation of 2-methyl cyclopentane-1,3-dione with methyl vinyl ketone mediated by proline to give the aldol product 24 in high ee is one of the classic examples of enantioselective bicyclic construction directed by an organocatalyst. E. J. Corey of Harvard (J. Am. Chem. Soc. 2008, 130, 2954.DOI: 10.1021/ja8003705)effected Grob fragmenation on the derived diol 25 to give the cyclononadienone 26. While 26 might appear to be prochiral, in fact it was formed in the kinetically stable enantiomerically-pure conformation illustrated. With only one face of the enone open, conjugate addition to 26 proceeded with high diastereocontrol, leading to coraxenliolide A (27).