Cruentaren A (3), isolated from the myxobacterium Byssovorax
cruenta, is an inhibitor of mitochondrial F-ATPase. The synthesis of 3
(Org. Lett. 2007, 9, 655,
DOI: 10.1021/ol0629317;
Angew. Chem. Int. Ed. 947725-04-4 uses 2007, 46, 5209.
DOI: 10.1002/anie.200701423)
by
Martin E. Rhodamine B isothiocyanate Formula Maier of the Universität Tübingen illustrates the power of alkyne
metathesis as a tool for the synthesis of complex natural products. Very
recently, Alois Fürstner of the Max-Planck-Institut, Mülheim, reported (Angew.
Chem. PMID:25269910 Int. Ed. 2007, 46, 9275.
DOI: 10.1002/anie.200703839)
an
alternative synthesis, also based on alkyne metathesis, of cruentaren A (3).
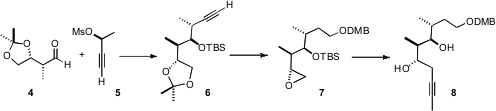
The alcohol portion of 1 was prepared by Marshall homologation of 4
with 5, leading to 6. Homologation of the derived epoxide 7
then gave 8. Note that the homologation of 7 to 8 required
three steps. This might have been accomplished more directly with the Li salt of
1-propyne, easily prepared from commercial 1- or 2-bromopropene.
Evans auxiliary-controlled homologation of 9 set the relative and
absolute configuration of 10, which was carried on to 12. To
effect coupling, the acid of 12 was activated with carbonyl diimidazole,
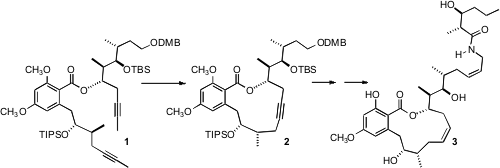
then condensed with the bis-alcoholate of 8. This acylation was highly
regioselective, giving 1 as the only observed product.
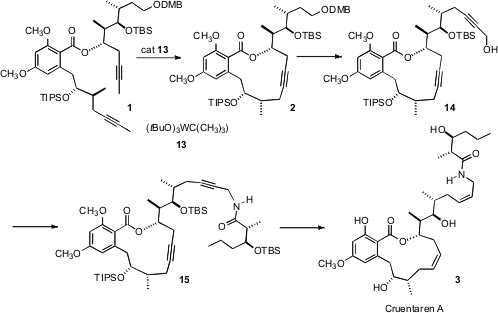
Cruentaren A (3) has two Z alkenes, so the authors chose a
bis-alkyne strategy, with a partial hydrogenation of both alkynes at the end of
the synthesis. To this end, alkyne metathesis was accomplished with the Schrock
tungsten carbine catalyst 13. Homologation to 15 followed by
deprotection and hydrogenation then gave enantiomerically pure cruentaren A (3).