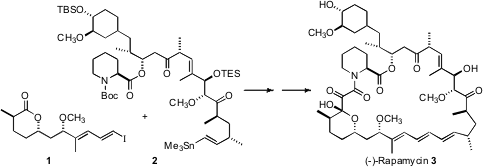
Rapamycin (3) is used clinically as an immunosuppressive agent. The synthesis of 3 (Angew. N-(2-Hydroxyethyl)maleimide Data Sheet Chem. Int. PMID:35345980 Morpholin-2-one site Ed. 2007, 46, 591.DOI: 10.1002/anie.200604053)by Steven V. Ley of the University of Cambridge was based on the assembly and subsequent coupling of the iododiene 1 and the stannyl alkene 2.
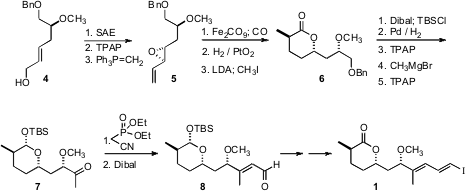
The lactone of 1 was prepared by Fe-mediated cyclocarbonylation of the alkenyl epoxide 5, following the protocol developed in the Ley group.
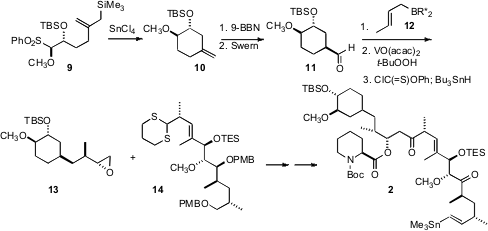
The cyclohexane of 2 was constructed by SnCl4-mediated cyclization of the allyl stannane 9, again employing a procedure developed in the Ley group. Hydroboration delivered the aldehyde 11, which was crotylated with 12, following the H. C. Brown method. The alcohol so produced (not illustrated) was used to direct the diastereoselectivity of epoxidation, then removed, to give 13. Coupling with 14 then led to 2.
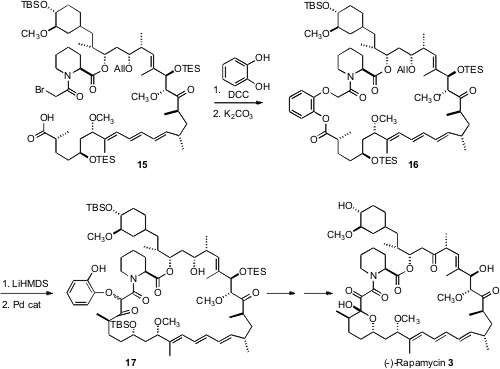
Combination of 1 with 2 led to 15, which was condensed with catechol to give the macrocycle 16. Exposure of 16 to base effected Dieckmann cyclization, to deliver the ring-contracted macrolactone 17, which was carried on to (-)-rapamycin (3).