A recent publication by the group of Phil S. Baran from the Scripps Research
Institute (Angew. Formula of 3-Vinylthiophene Chem. Int. Ed. PMID:24268253
2004, 43, 2674.
DOI: 10.1002/anie.200453937)
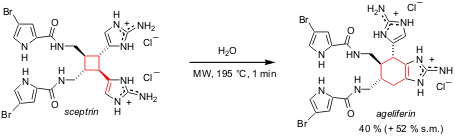
reports the total synthesis of ageliferin, an antiviral agent with
interesting molecular architecture. Just one minute (!) of microwave irradiation
of sceptrin, another natural product, for 195 °C under sealed vessel conditions
provides ageliferin in 40% yield, along with 52% of recovered starting material.
Remarkably, if the reaction is performed without microwaves at the same
temperature only starting material and decomposition products are observed. 3-Bromo-2-iodobenzo[b]thiophene structure
Diels-Alder Cycloaddition Chemistry
Christopher J. Moody and co-workers from the University of Exeter have
employed (Chem. Commun. 2004, 946.
DOI: 10.1039/b401580k) a "biomimetic" hetero-Diels-Alder-aromatization sequence for the
construction of the pyridine ring in amythiamicin D. The key cycloaddition
reaction between the azadiene and enamine component was carried out by microwave
irradiation at 120 °C for 12 hours (see also
Chem. Commun. 2002, 1760.
DOI: 10.1039/b204868j)
and gave the required 2,3,6-tris(thiazolyl)pyridine intermediate in
moderate yield. Coupling of the remaining building blocks then completed the
first total synthesis of the thiopeptide antibiotic amythiamicin D.

Suzuki-Miyaura Cross-Coupling Reactions
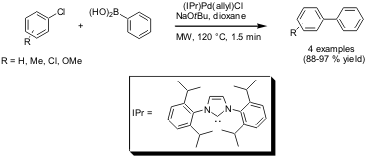
A series of air- and moisture-stable (N-heterocyclic
carbene)Pd(allyl)Cl complexes has been shown by Steven P. Nolan and coworkers (J.
Org. Chem. 2004, 69, 3173.
DOI: 10.1021/jo035834p)
to catalyze Suzuki-Miyaura cross coupling reactions of aryl chlorides
with boronic acids. This catalytic system is compatible to microwave conditions
and rapid couplings were observed within 1.5 min at 120 °C. The conventionally
heated reactions (60 °C) required several hours to reach completion. The same
article also reports on microwave-assisted dehalogenations of aryl chlorides
using the same catalytic system.
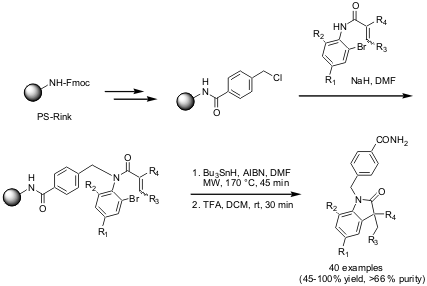
Solid-Phase Synthesis of Indol-2-ones via Radical Cyclization
Koichi Fukase and coworkers from Osaka University have reported (Synlett
2004, 1049.
DOI: 10.1055/s-2004-820052)
the solid-phase synthesis of indol-2-ones (4 diversity points) via a
radical cyclization pathway. The key cyclization step was carried out by using
Bu3SnH and AIBN in DMF under microwave irradiation conditions,
providing a small library of 40 compounds. Interestingly, the related radical
cyclization in solution phase was considerable less effective.
Review and Feature Articles
Two recent review articles highlight the importance of microwave chemistry
for carbohydrate chemistry:
S. K. Das, "Application of Microwave Irradiation in the Synthesis of
Carbohydrates",
Synlett 2004, 915-932.
DOI: 10.1055/s-2004-820034
A. Corsaro, U. Chiacchio, V. Pistara, G. Romeo,
"Microwave-Assisted Chemistry of Carbohydrates",
Curr. Org. Chem. 2004, 8, 511-538.
Available for free
(Reproduced by permission of
Bentham Science Publishers)