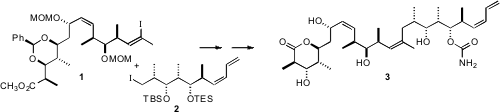
(+)-Discodermolide (3), a potent anticancer agent that works synergistically with taxol, may yet prove to be clinically effective. For the synthetic material to be affordable, a highly convergent synthesis is required. Jean-François Betzer and Janick Ardisson of the Université de Cergy-Pontoise have described (Angew. Chem. Int. PMID:24220671 Price of 1217603-41-2 Ed. 2007, 46, 1917. DOI: 10.1002/anie.200604629)such a synthesis, coupling 1 and 2. A central feature of their approach was the repeated application of the inherently chiral secondary organometallic reagent 5.
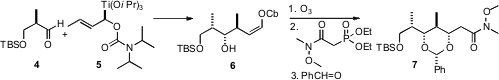
The first use of 5 was the addition to the aldehyde 4. (R)-4-tert-Butyl-2-oxazolidinone Formula The product 6 was ozonized, and the resulting aldehyde was carried on to the α,β-unsaturated ester. Exposure of the hydroxy ester to benzaldehyde under basic conditions delivered, by intramolecularMichael addition, the acetal 7.
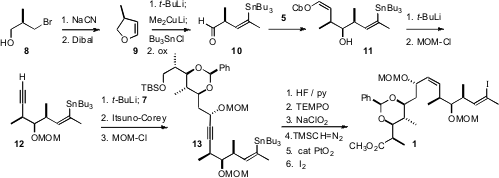
The next addition of the reagent 5 was to the aldehyde 10. The adduct11 was deprotonated with t-BuLi to effect α-elimination, providing, after protection of the alcohol, the alkyne 12. Coupling of 12 with the amide 7 gave a ketone, enantioselective reduction of which underItsuno-Corey conditions led, again after protection of the alcohol, to the alkyne 13. Oxidation followed by selective hydrogenation and iodine-tin exchange then completed the assembly of 1. Note that PtO2, not typically used for partial hydrogenation, was the catalyst of choice for this congested alkyne.
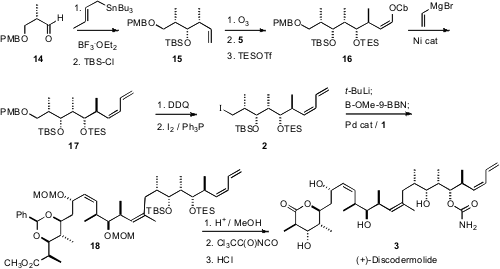
The third application of the enantiomerically-pure reagent 5 was addition to the aldehyde that had been prepared by ozonolysis of 15. Advantage was then taken of another property of the alkenyl carbamate, Ni-mediated Grignard coupling, to form the next carbon-carbon bond with high geometric control. Deprotection of the diene 17 so prepared followed by iodination then completed the synthesis of 2.
The convergent coupling of 1 with 2 was carried out underSuzuki conditions. Reduction of the iodide of 2 to the corresponding alkyl lithium followed by exchange with B-OMe-9-BBN gave gave an intermediate organoborane, that smoothly coupled with 1 under Pd catalysis to give 18. Deprotection and carbamate formation then led to (+)-discodermolide (3).
This synthesis clearly illustrates the power of 5 as an enantiomerically-defined secondary organometallic reagent, and the synthetic versatility of the product alkenyl carbamates. The ready availability of the three enantiomerically-pure four-carbon fragments 4, 8, and 14 was also a key consideration in the design of this synthesis.