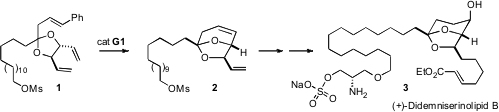
The sulfate (+)-didemniserinolipid B (3), isolated from the tunicateDidemnum sp, has an intriguing spiroether core. 1-Bromo-2-fluorobenzene Chemscene A key step in the synthesis of 3 reported (Org. PMID:25046520 Lett. 1031967-52-8 site 2007, 9, 5357.DOI: 10.1021/ol702454m)by Steven D. Burke of the University of Wisconsin was the selective ring-closing metathesis of 1 to 2.
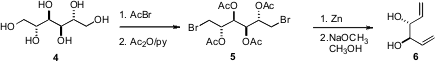
The diol 6 that was used to prepare the ketal 1 was readily prepared from the inexpensive D-mannitol (4). Many other applications can be envisioned for the enantiomerically-pure diol 6 and for the monoacetate and bis acetate that are precursors to it.
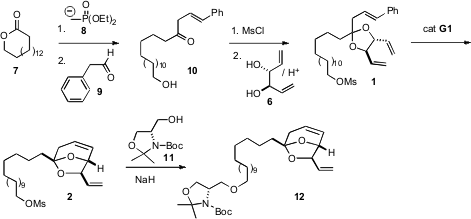
To set up the metathesis, the β,γ-unsaturated ketone 10 was needed. To this end, the keto phosphonate derived from the addition of the phosphonate anion 8 to the lactone 7 was condensed with phenyl acetaldehyde 9. The derived enone10 was a 5:1 mixture of β,γ- and α,β- regioisomers.
The diol 6 is C2-symmetrical, but formation of the ketal 1 dissolved the symmetry, with one terminal vinyl group directed toward the styrene double bond, and the other directed away from it. On exposure to the first generation Grubbs catalyst, ring formation proceeded efficiently, to give2. Williamson coupling with the serine-derived alcohol 11 then gave 12.
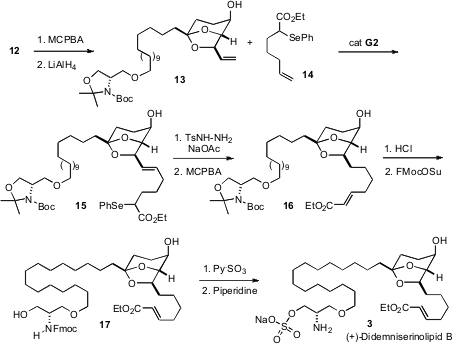
To establish the secondary alcohol of 13 and so of 3, the more electron rich alkene of 12 was selectivelyepoxidized, from the more open face. Diaxial opening with hydride then gave 13.
With 13 in hand, another challenge of selectivity emerged. The plan had been to attach the ester-bearing sidechain to 13 usingalkene metathesis, then hydrogenate. As the sidechain of 3 contained an additional alkene, this had to be present in masked form. To this end, the α-phenylselenyl ester 14 was prepared. Alkene metathesis with13 proceeded smoothly, this time using the second generation Grubbs catalyst. The unwanted alkene was then removed by reduction with diimide, and the selenide was oxidized to deliver the α,β-unsaturated ester.