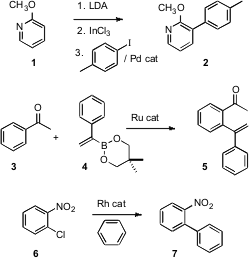
The direct functionalization of a C-H bond is a powerful transformation for organic synthesis, allowing the rapid elaboration of desired complexity from inexpensive starting materials. Formula of (3-Hydroxy-5-methylphenyl)boronic acid Even Friedel-Crafts acylation can be seen as a C-H functionalization reaction, from the point of view of the aromatic ring, and aromatic substitution continues to dominate efforts toward C-H functionalization. 2-Aminopropanenitrile hydrochloride Price While the directed metalation of aromatic rings has been known for some time, carbon-carbon bond formation at the metalated site has sometimes been problematic. José Pérez Sestelo and Luis A. Sarandeses of the Universidade da Coruña, Spain, have now shown (J. Org. Chem. 2007, 72, 1271. DOI: 10.1021/jo062148s)that addition of the intermediate metalated aromatic to InCl3 gives an intermediate that couples efficiently to aryl halides under Pd catalysis. Vinyl triflates also work well. In a recent advance in catalytic ortho metalation, Fumitoshi Kakiuchi of Keio University has developed (J. Org. Chem. 2007, 72, 3600.DOI: 10.1021/jo070182g)conditions for in situ coupling of the metalated intermediate to an alkenyl boronate. PMID:24190482 The C-H to be functionalized can be on another ring. Rhett Kempe of the Universität Bayreuth has uncovered (Angew. Chem. Int. Ed. 2007,46, 3135.DOI: 10.1002/anie.200604988)a Rh catalyst that will couple haloaromatics such as 6 directly to solvent benzene.
Intramolecular C-C bond formation by C-H functionalization is also a powerful transformation. Alexei V. Novikov of the University of North Dakota has devised (Org. Lett. 2007, 9, 61.DOI: 10.1021/ol062592h)a new sulfonate tether, exemplified by 8. As the sulfonate can be removed reductively, the net transformation is the C-2 homologation of a C-H directed by a distal OH.
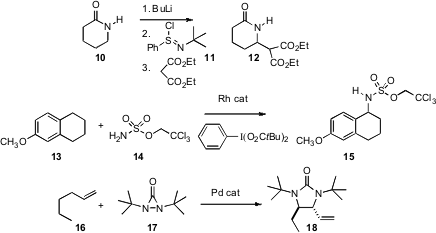
There have been several recent developments around amine-centered bond formation. Jun-ichi Matsuo and Hiroyuki Ishibashi of Kanazawa University have used (Tetrahedron Lett. 2007, 48, 3233.DOI: 10.1016/j.tetlet.2007.03.025)the commercial reagent 11 to oxidize the lactam 10 to the intermediate iminium species. Alkyl malonates could then be added directly, to give the C-H alkylated product 12. Both Justin Du Bois of Stanford University (J. Am. Chem. Soc. 2007, 129, 562.DOI: 10.1021/ja0650450)and Hélène Lebel of the Université de Montréal (Org. Lett. 2007, 9, 639. DOI: 10.1021/ol062953t)have developed reagent and catalyst combinations for direct C-H amination. In one of the more spectacular recent developments, Yian Shi of Colorado State University has established (J. Am. Chem. Soc. 2007, 129, 7496.DOI: 10.1021/ja072080d)conditions for the diastereoselective activation of two C-H bonds, adding 17 to terminal alkenes to give the protected diamine 18.
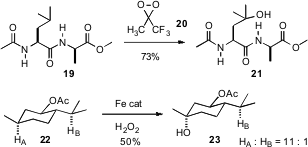
The reagent of choice for C-H hydroxylation has been methyl(trifluoromethyl)dioxirane 20, as illustrated by the recent work of Paul G. Williard of Brown University (J. Org. Chem. 2007, 72, 525. DOI: 10.1021/jo061910n). M. Christina White of the University of Illinois has recently devised (Science2007, 318, 718.DOI: 10.1126/science.1148597)an Fe complex that catalytically effected C-H hydroxylation. This reagent showed substantial (probably steric) selectivity. It will be interesting to learn in what ways this new catalyst and 20 are complementary.