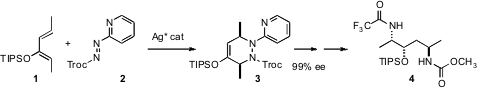
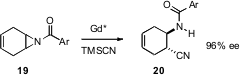A classic strategy for controlling relative stereocontrol is to imbed the
stereogenic centers in a ring. PMID:23415682 Hisashi Yamamoto of the University of Chicago has
developed (J. Am. Chem. Soc. 2006, 128, 16482.
DOI: 10.1021/ja066726y)
a silver catalyst that directs
the absolute sense of the
Diels-Alder cyclization of the dienophile 2. The
adduct 3 was easily unraveled to the 1,4-diamine 4.
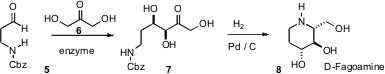
The enzyme-mediated addition of 1,3-dihydroxy acetone (6) to an aldehyde is an
attractive strategy for the rapid elaboration of carbohydrates. This approach
has been limited, however, by the requirement that 6 first be converted to the
phosphate. Jesús Joglar and Pere Clapés of the Institute for Chemical and
Environmental Research, Barcelona, have found (Org. Lett. 2006, 8, 6067.
DOI: 10.1021/ol0625482)
that
crude recombidant fructose-6-phospate aldolase works well with 6, adding it to
5
to give 7 in high ee. The triol 7 was reduced to D-fagomine (8), N-alkyl
derivatives of which are inhibitors of α-D-glucosidase.
Several years ago (J. Am. Chem. 3-Methoxy-4-pyridinamine manufacturer Soc. 1998, 120, 11798.
DOI: 10.1021/ja981075u), Nicos A. Fmoc-Bip(4,4′)-OH web Petasis of
the University of Southern California described a
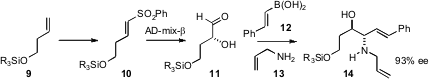
borono-Mannich homologation of chiral α-hydroxy aldehydes such as 11. The limitation on this was the difficulty
of preparing the α-hydroxy aldehydes. Stephen G. Pyne of the University of
Wollongong has now devised (J. Org. Chem. 2006, 71, 7097.
DOI: 10.1021/jo0610661)
a scheme for the
enantioselective homologation of a terminal alkene 9 using the Petasis procedure.
The alkene was converted into the unsaturated sulfone 10 either by Ru-catalyzed
metathesis or, more economically, by iodosulfonylation followed by elimination.
Exposure of the unsaturated sulfone to AD-mix-β (or α) gave the crude α-hydroxy
aldehyde 11, which as carried on directly via the Petasis protocol to give
14.
Viresh H. Rawal, also of the University of Chicago, has made (Angew. Chem.
Int. Ed. 2006, 45, 6130.
DOI: 10.1002/anie.200601638)
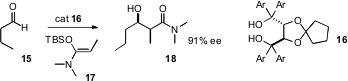
the remarkable observation that the taddol
16,
originally designed as a ligand for early transition metals, is an effective
organocatalyst for the
Mukaiyama aldol
reaction of 17 with aldehydes.
Professor Rawal has also found that the product tertiary amides can be
reduced
with Cp2Zr(H)Cl to the aldehydes with little or no epimerization.
A powerful strategy for the assembly of enantiomerically-pure fragements is
the desymmetrization of meso precursors. Masakatsu Shibasaki of the University
of Tokyo has designed (J. Am. Chem. Soc. 2006, 128, 16438.
DOI: 10.1021/ja067003h)
a chiral Gd catalyst
for the opening of meso aziridines such as 19 to the amido nitrile
20 in high ee.
Much good work has been reported recently on the enantioselective
construction of contiguous alkylated stereogenic centers. One of the most
powerful approachs is the organocatalyst-mediated hetero
Diels-Alder
condensation developed (J. Am. Chem. Soc. 2006, 128, 8418.
DOI: 10.1021/ja062707c)
by Jeffrey W. Bode of
the University of California, Santa Barbara. More recently, Professor Bode
reported (J. Am. Chem. Soc. 2006, 128, 15088.
DOI: 10.1021/ja066380r)
that the analogous oxa reaction,
to make cyclic ethers, also works well.
Attention has been focused by several groups on the chiral
organocatalyst-mediated conjugate addition of aldehydes to unsaturated
nitroalkenes. Claudio Palomo of the Universidad del País Vasco has optimized (Angew.
Chem. Int. Ed. 2006, 45, 5984.
DOI: 10.1002/anie.200602207)
additions such as that of
15 to 24. Eric N.
Jacobsen of Harvard University has used (Angew. Chem. Int. Ed. 2006, 45, 6366.
DOI: 10.1002/anie.200602221)
a related approach to prepare quaternary alkylated stereogenic centers in high ee.