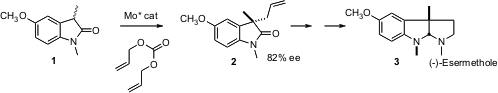
Barry M. Trost of Stanford University has developed powerful methods for catalytic enantioselective allylation. In a recent application (J. BuyPerfluoropropionic anhydride Am. Chem. Soc. 2006, 128, 4590. DOI: 10.1021/ja060560j)he has applied this approach toward the preparation of the physostigmine alkaloids. Allylation of 1 proceeded in high ee to give 2, which was carried on to (-)-esermethole (3). Other oxindoles were allylated in up to 95% ee.
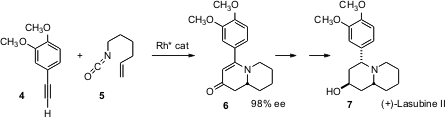
Tomislav Rovis of Colorado State University has reported (J. Am. Chem. Soc. 2006, 128, 12370. DOI: 10.1021/ja064868m)a particularly powerful method for the construction of fused amines such as 6, by the enantioselective condensation of alkynes with unsaturated isocyanates such as 5. The vinylogous amide 6 was readily carried on to (+)-lasubine II (7). PMID:23880095
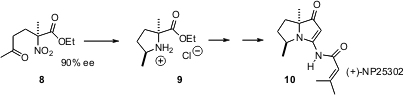
Using (J. Org. Chem. 220497-67-6 structure 2006, 71, 8579. DOI: 10.1021/jo061650+)a protocol developed by Li Deng of Brandeis University, Barry B. Snider, also of Brandeis, added ethyl 2-nitropropionate to methyl vinyl ketone to give 8 with high ee. Reductive cyclization proceeded with 95:5 diastereocontrol, leading to 9 and thus to natural (+)-NP25302 (10). This study established the absolute configuration of 10.
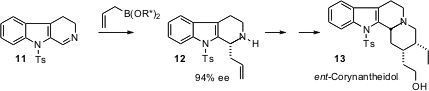
Cyclic imines such as 11 are easily prepared. J. Michael Chong of the University of Waterloo has now found (J. Am. Chem. Soc. 2006, 128, 9646. DOI: 10.1021/ja0636791)that BINOL-derived allylboronates add to such imines to give the cyclic amines such as 12 in high ee. The conversion of 12 to ent-corynantheidol (13) involved a very interesting diastereoselectiveMichael addition.
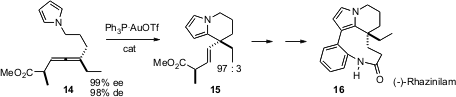
Starting with the enantiomerically-pure allene 14, prepared by the methods he had previously developed, Scott G. Nelson of the University of Pittsburgh established (J. Am. Chem. Soc. 2006, 128, 10352. DOI: 10.1021/ja0629110)that an Au catalyst effected cyclization onto the electron-richpyrrole with high diastereoselectivity. This set the stage for the enantioselective synthesis of (-)-rhazinilam 16. The pendant methyl of 15 served as an internal reporter, making it easy to follow the stereochemical course of the cyclization.
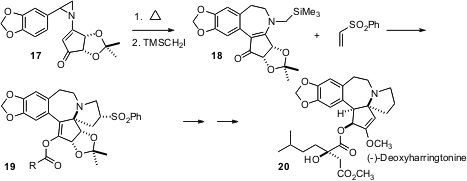
David Y. Gin, now at the Memorial Sloan-Kettering Cancer Center in New York, has been exploring (J. Am. Chem. Soc. 2006, 128, 10370. DOI: 10.1021/ja063304f)ring-strain releasing rearrangment of N-vinyl 2-aryl aziridines such as 17. Thermal rearrangment followed by alkylation with Me3SiCH2I gave 18. Sequential O-acylation and exposure to fluoride ion gave a 1,3-dipole, that added to phenyl vinyl sulfone with high diastereocontrol to give 19. This was carrried on to the potent anti-leukemia alkaloid (-)-deoxyharringtonine (20).