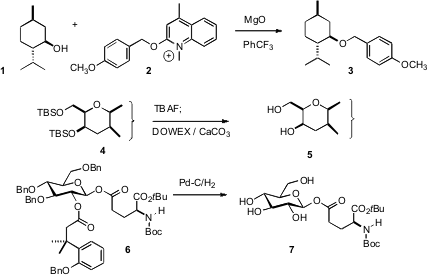
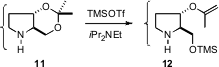There have been several useful developments in alcohol protection. Gregory B. Dudley of Florida State University has devised (Chem. Commun. 6-Bromo-5-fluoronicotinaldehyde Chemscene 2007, 1436.DOI: 10.1039/b617926f)a new reagent 2 for the preparation of p-methoxybenzyl (PMB) ethers. Yoshito Kishi of Harvard University, faced with the difficulty of isolating a deprotected natural product via aqueous work-up, found (Org. Lett. 2007, 9, 723.DOI: 10.1021/ol063113h)that the THF/TBAF reaction mixture could be mixed with a sulfonic acid ion exchange resin and CaCO3, then simply filtered and evaporated. Formula of 87789-35-3 Benzyl ethers are commonly removed by hydrogenation at the end of a synthesis. David Crich, now at Wayne State University, desiring (Org. Lett. 2007, 9, 1613.DOI: 10.1021/ol070449y)to simultaneously remove an ester, created the protecting group illustrated in 6. Hydrogenolysis followed by lactone formation liberated the free OH. PMID:23613863
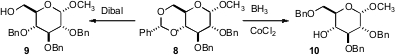
The protection of diols is also important. Usually, reduction of benzylidene protected diols such as 8 would lead to the primary benzyl ether 9. Ken-ichi Sato of Kanagawa University has developed (Tetrahedron Lett. 2007, 48, 3103.DOI: 10.1016/j.tetlet.2007.02.133)conditions for the complementary reduction, to give selectively the secondary benzyl ether 10. Larry E. Overman of the University of California, Irvine, applying a procedure originally developed by Scott D. Rychnovsky, found (Org. Lett. 2007, 9, 339.DOI: 10.1021/ol062801y)that the acetonide11 could be converted selectively into 12.
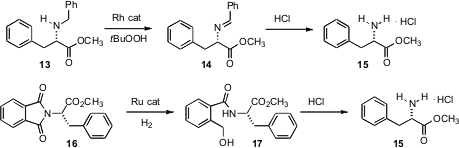
Nitrogen protecting groups can be removed both oxidatively and reductively. Michael P. Doyle of the University of Maryland has found (Chem. Commun. 2007, 745. DOI: 10.1039/b615805f)that Rh2(caprolactamate)4 mediates the oxidation of benzylamines such as 13 to the imine 14. Exposure to HCl then gave the amine salt 15. Takao Ikariya of Tokyo Institute of Technology has devised (J. Am. Chem. Soc. 2007, 129, 290. DOI: 10.1021/ja067777y)a Ru catalyst that is active for the hydrogenation of imides such as the phthalimide 16. Exposure to HCl deprotected 17 to give 15.
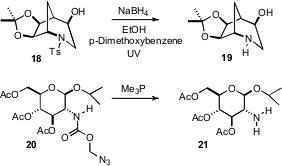
Although Ts is a convenient and robust protecting group for amines, it is sometimes difficult to remove. Stephen G. Bergmeier of Ohio University observed (J. Org. Chem. 2007, 72, 1024.DOI: 10.1021/jo0619231)that dissolving metal deprotection of 18 gave a complex mixture, but that the Yonemitsu procedure (J. Am. Chem. Soc. 1986, 108, 140) worked well. Extending the array of N protecting groups, Nicolas Winssinger of the Université Louis Pasteur has explored (Org. Lett. 2007, 9, 2223.DOI: 10.1021/ol0707160)the azidomethyl (Azoc) carbamate 20. The Azoc group is compatible with the reagents to remove Fmoc (piperidine) and Mtt (TFA). The conditions for Azoc removal, brief exposure to a phosphine (which can be polymer bound), are compatible with many other protecting groups.