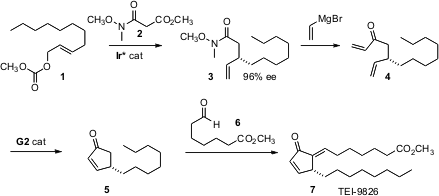
TEI-9826 (7) is a prostaglandin analogue that shows high activity against cisplatin-resistant tumors. 457613-78-4 Order Günter Helmchen of the Universität Heidelberg has devised (Angew. Chem. Int. Ed. PMID:29844565 817562-90-6 supplier 2006, 45, 2466. DOI: 10.1002/anie.200503945)a simple but elegant route to 7, based on the Ir*-catalyzed condensation of 2 with the allylic carbonate 1. Remarkably, the easily-epimerized stereogenic center of 5 survives not just metathesis, but also the alkaline conditions of the aldol reaction used to attach the upper sidechain.
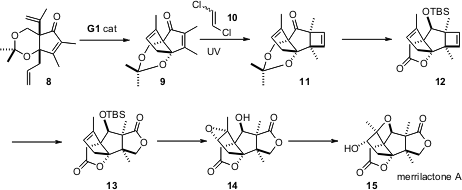
The pentacyclic merrilactone A (15) is a potent neurotropic agent, promoting neurite outgrowth in cortical neuron cell culture. In the synthesis of15 by Goverdhan Mehta of the Indian Institute of Science, Bangalore (Angew. Chem. Int. Ed. 2006, 45, 953. DOI: 10.1002/anie.200503618)alkene metathesis is deployed early, in the transformation of 8 to 9 that establishes the second of the two carbocyclic rings.
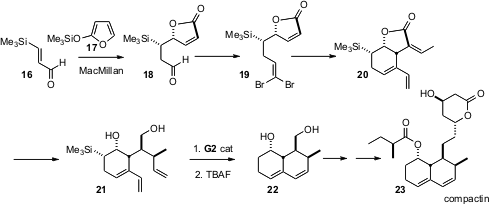
The aptly-named compactin (23), the parent of the clinically-important statin cholesterol-lowering agents, indeed has a compact carbocyclic core. The formal synthesis (Org. Lett. 2006, 8, 597. DOI: 10.1021/ol0527328)of 23 by Joël Robichaud of Merck Frosst Canada, delivers, with control of relative and absolute configuration, the diol 22. Initial stereocontrol is achieved by the addition of 17 to 16, using the MacMillan protocol. Free radical cyclization of the dibromide 19 leads to the cyclic bromoalkene, which is homologated with vinyl tributyl tin to give the diene. Conjugate addition to the exocyclic alkene of 20 again proceeds with high diasterocontrol, to deliver 21, the substrate for alkene metathesis.
In the preparation of the antibiotic kendomycin (30) by Amos B. Smith III of the University of Pennsylvania (J. Am. Chem. Soc. 2006, 128, 5292. DOI: 10.1021/ja060369+), the metathesis substrate 27 was assembled using a triply convergent strategy, combining 24, 25, and 26. The influence of the substituents on the conformation of the incipient medium ring were such that only one of the two diastereomers of the alcohol 27 would cyclize. The product 28 was the undesired Z alkene, fortunately exclusively, so it could be inverted by cis dihydroxylation followed by stereocontrolled epoxide deoxygenation.